Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Carboxylic acids and derivatives – solutions to the problems.
Solution 1:
The acidity in carboxylic acids increases with the presence of electron-attracting groups close to the carboxyl group, since on the one hand they weaken the O-H bond, which helps to release a proton, and on the other hand they stabilize the carboxylate ion, dispersing the negative charge between the two oxygen atoms of the carboxylate ion and making it more stable. The higher the electronegativity of the groups, the greater their number and the closer they are to the carboxyl group, the higher the acidity. On the other hand, alkyl groups are electron-donating, so their presence makes it difficult to break the O-H bond. According to these criteria the order of acidity will be as follows for the different series:
- a) HCOOH > CH3-COOH > CH3-CH2-COOH
- b) CCl3-COOH > CHCl2-COOH > CH2Cl-COOH > CH3-COOH
- c) CH2F-COOH > CH2Cl-COOH > CH2Br-COOH
- d) CH3-CHCl-COOH > CH2Cl-CH2-COOH > CH3-CH2-COOH
Solution 2:
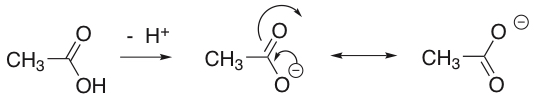
Solution 3:
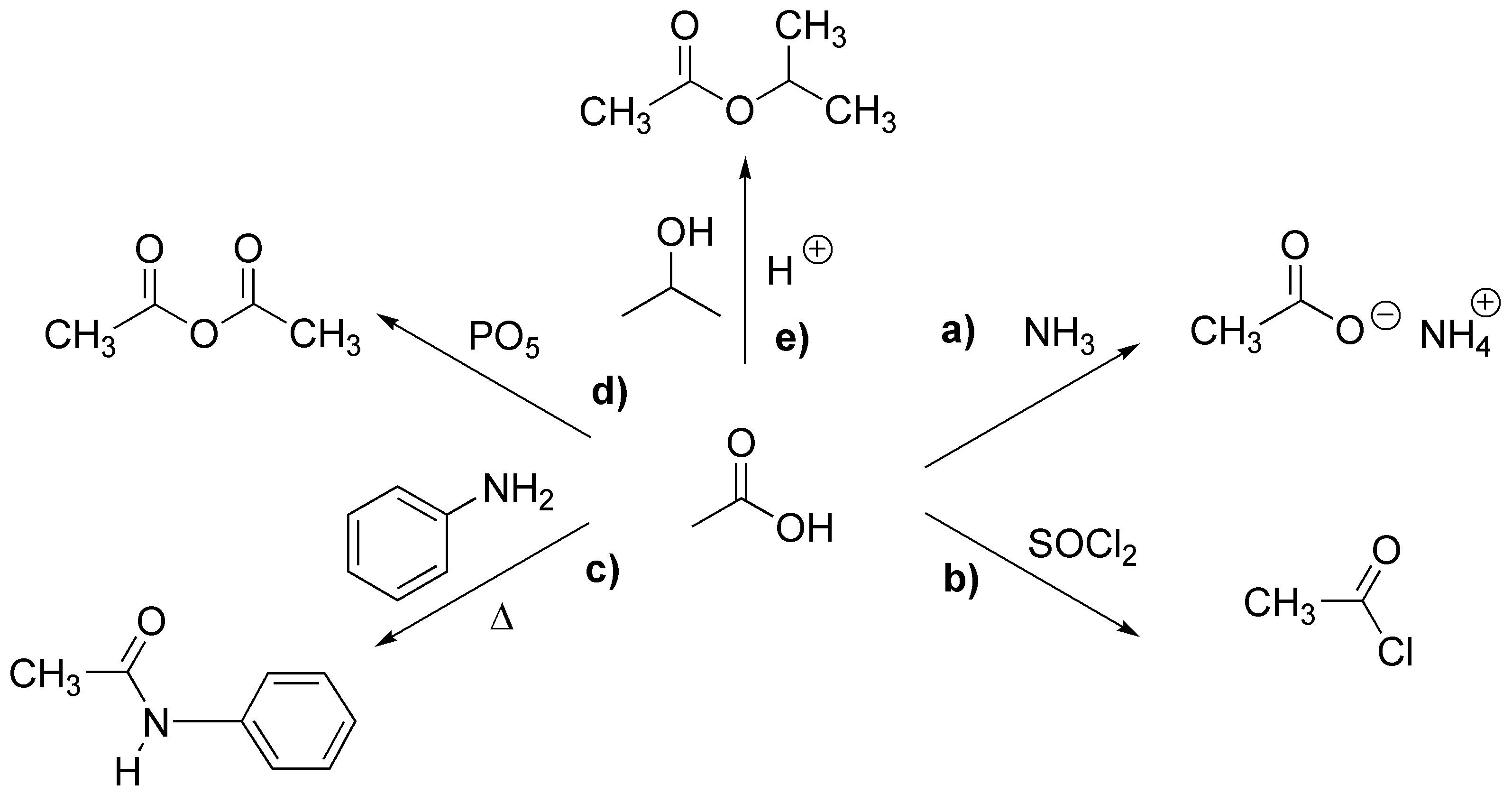
Solution 4:
The sterefication reaction is an equilibrium process that proceeds by the following mechanism:
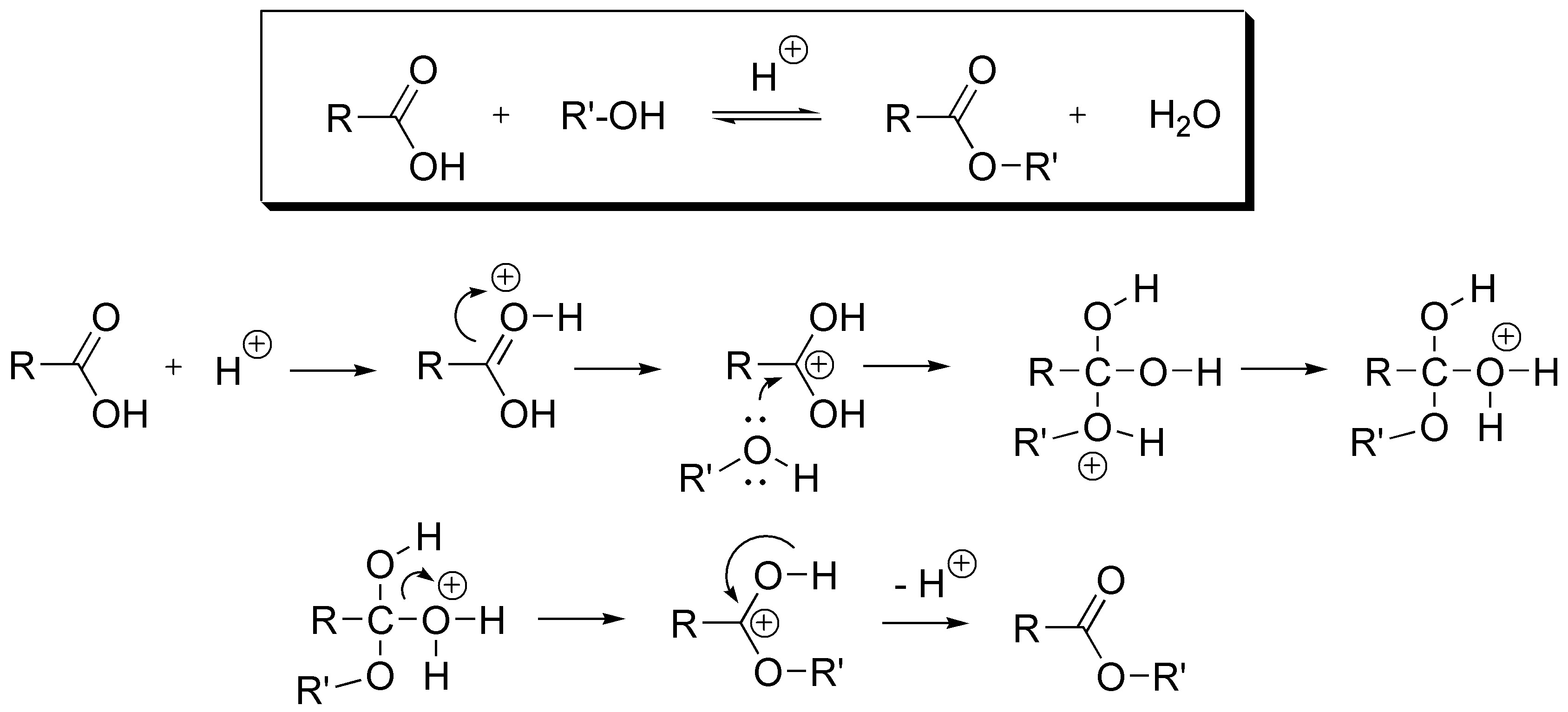
To shift the equilibrium to the right, a dehydrating agent can be used to remove the water, thereby shifting the equilibrium toward ester formation. Or, if the physical properties of the reaction components permit, the ester formed can be removed from the reaction crude. All this is done by a distillation process if the boiling point of the ester is lower than that of the acid and the starting alcohol.
Solution 5:
The reaction is a trans-esterification:

Solution 6:
The amide will preferentially be formed whenever there is a base in the reaction medium that traps the HCl that is released. The nitrogen atom is more nucleophilic than oxygen, but if there is no base in the medium, such as pyridine or triethylamine, the nitrogen of the 4-aminobutan-1-ol will be protonated in the course of the reaction forming the corresponding ammonium salt, which makes the electron pair of the nitrogen unavailable and it will then be the -OH group that will attack the carbon of the acid chloride.
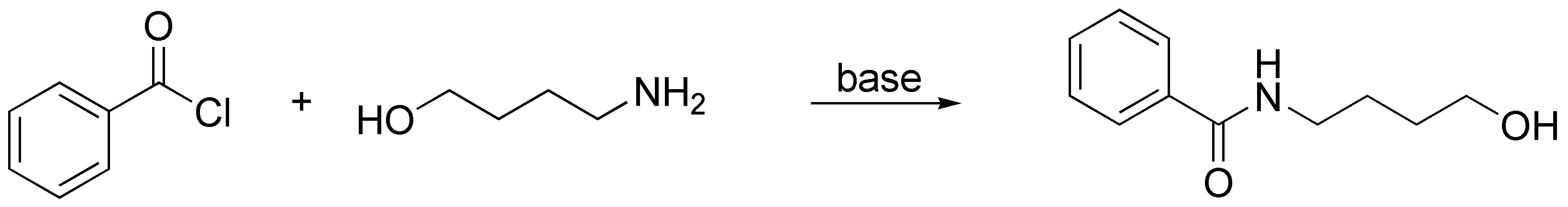
Solution 7:
Anhydrides react with both alcohols and amines, but with amines the reaction is faster as they are more nucleophilic than alcohols.
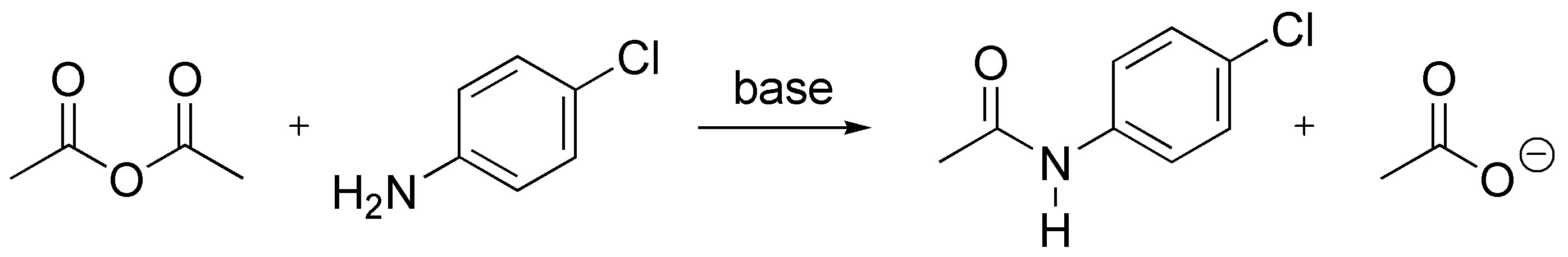
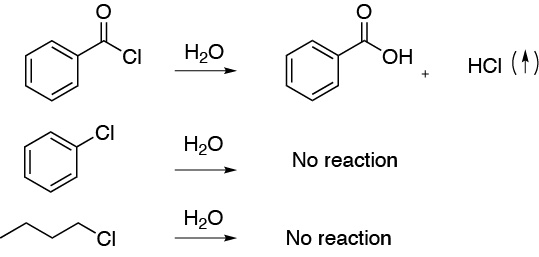
Solution 8:
a) Acetyl chloride reacts violently with water producing HCl vapors, while chlorobenzene and chlorobutane do not react. In this way we will have identified the first bottle.
b) To distinguish between chlorobenzene and chlorobutane, we treat with a sodium hydroxide solution. Chlorobenzene is inert at these conditions, since the chlorine attached to the aromatic ring cannot be substituted by HO–, while the chlorine atom of chlorobutane is relatively easily substituted, since it is a primary alkyl halide. We neutralize both solutions and add a few drops of silver nitrate solution. The appearance of a precipitate denotes the presence of chloride ion for the chlorobutane sample.
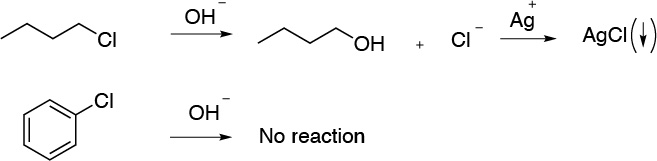
Solution 9:
The result is the formation of acetylsalicylic acid (aspirin). Acetic acid is obtained as a by-product. The reaction is catalyzed by addition of a few drops of mineral acid such as sulfuric acid or phosphoric acid.
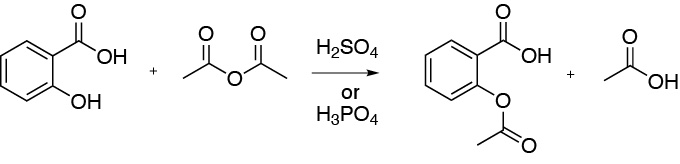
Solution 10:
Cycle opening occurs and 3-(ethylcarbamoyl)propanoyl acid is obtained.
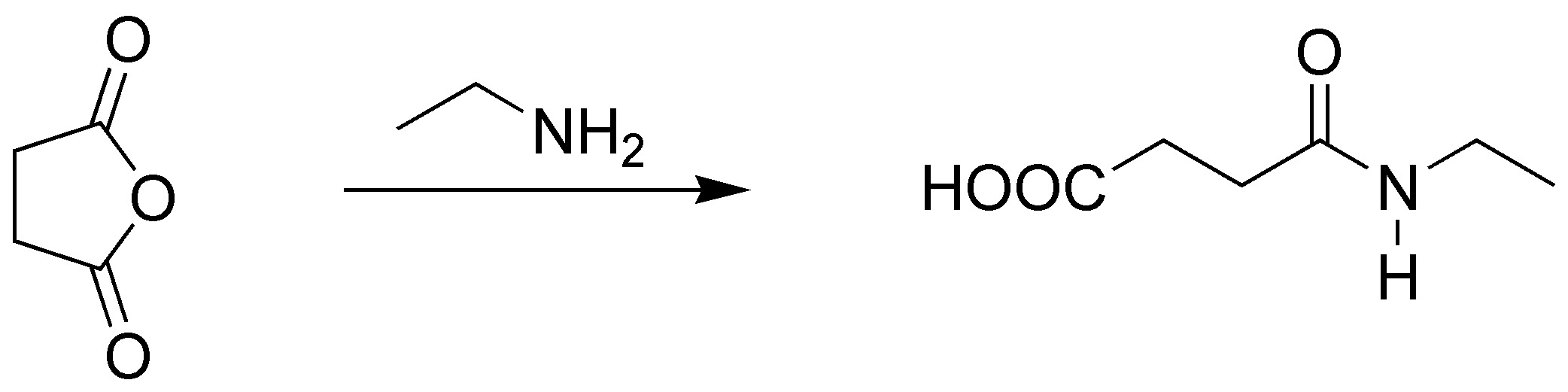
Solution 11:
By treatment with an amine the esters are transformed into amides.
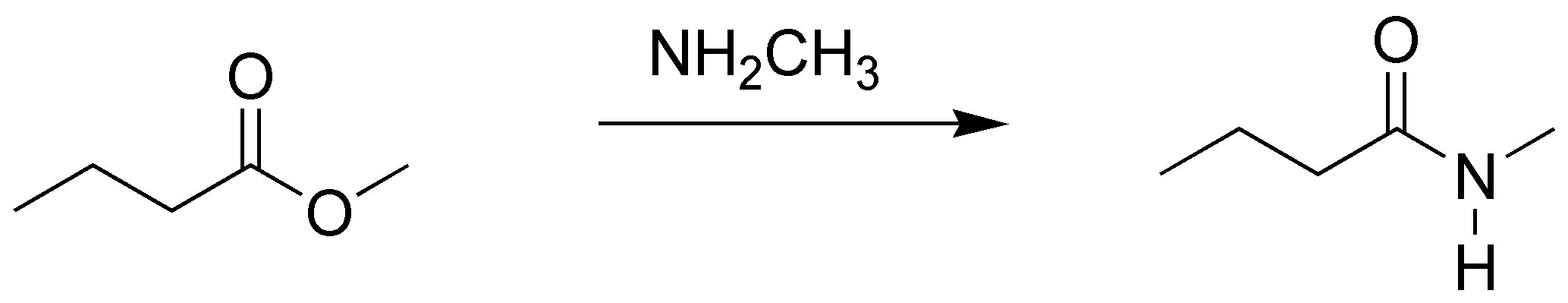
Solution 12:
Under acid catalysis conditions (HCl) the corresponding a) ethanoic and b) propanoic acids as well as a) ethyl and b) methyl alcohols are obtained, respectively. In basic catalysis (NaOH) the carboxylic acids would form the corresponding sodium salts, a) sodium ethanoate and b) sodium propanoate. Note that the acid catalyzed reaction is equilibrium while the basic one is not.
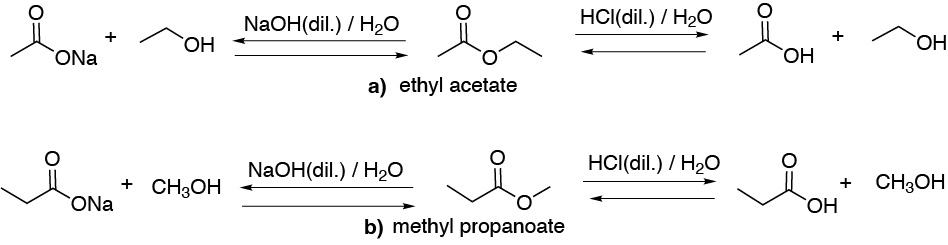
Solution 13:
The reactant is a fat (glycerol triester), which under basic conditions hydrolyzes into 3 molecules of the sodium salt of the corresponding carboxylic acid and glycerol. This reaction is called saponification and the sodium salt of fatty acids is used as soap.
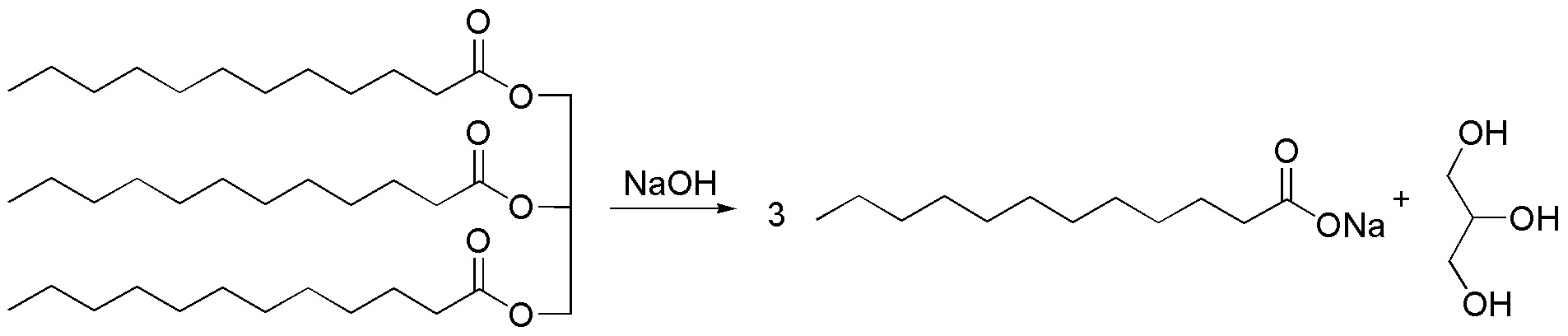
Solution 14:
- a) the corresponding acid halide is obtained.
- b) in basic medium the acid is deprotonated to give the carboxylate ion.
- c) using CH2N2 with an acid gives the corresponding methyl ester.
- d) is an esterification reaction giving the ethyl ester shown in the figure.
- e) carboxylic acids are reduced to alcohol using aluminum lithium hydride, in this case a secondary alcohol is generated.
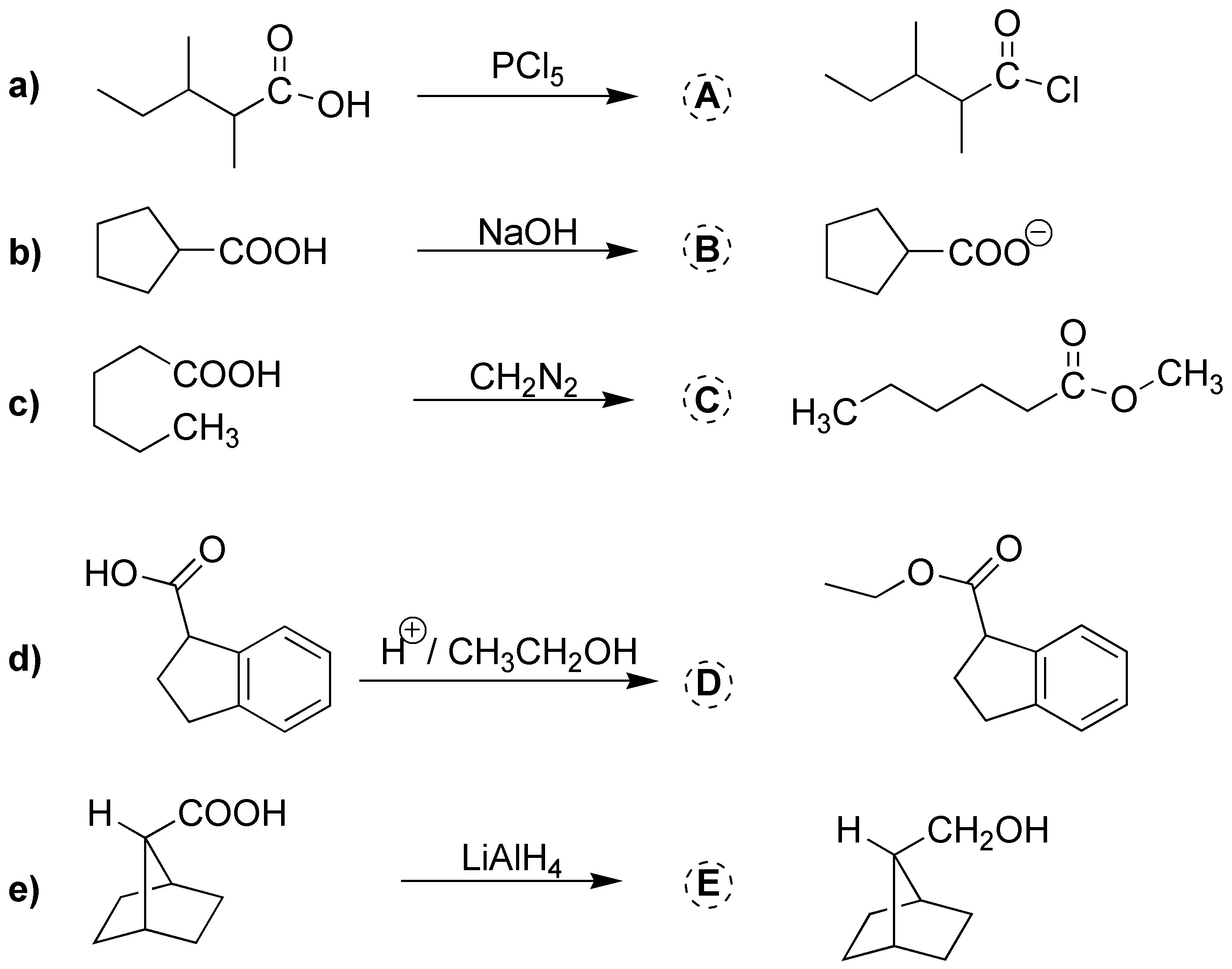
Solution 15:
The acid bromide can be obtained by employing PBr3. b) The reduction to alcohol is carried out with LiAlH4. c) The esterification is carried out with the alcohol in the figure in acidic medium. d) To generate the carboxylate anion, basic medium is employed. e) The methyl ester is obtained by using diazomethane, CH2N2.
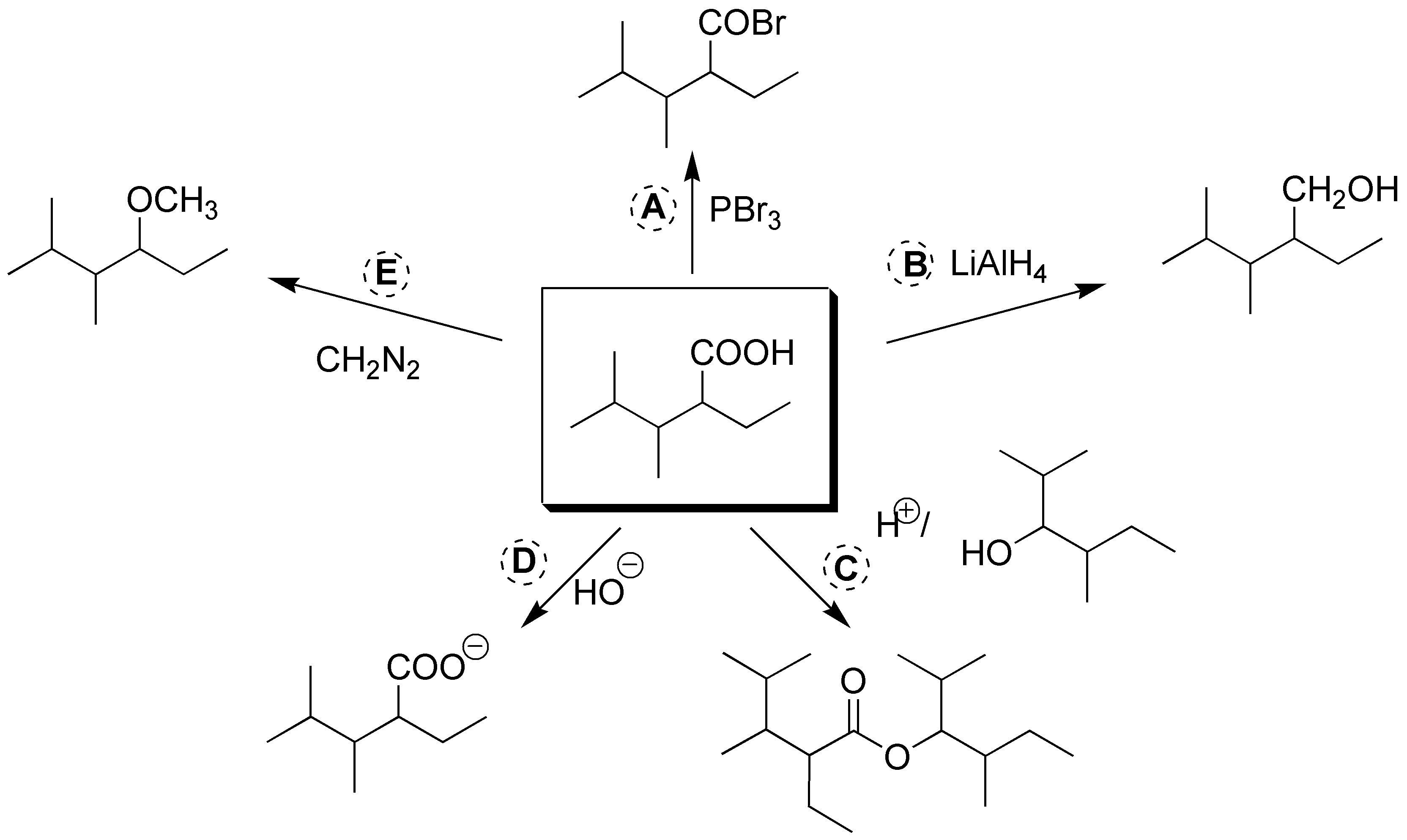
Solution 16:
The reagents and products needed to complete the scheme are shown in the figure below:
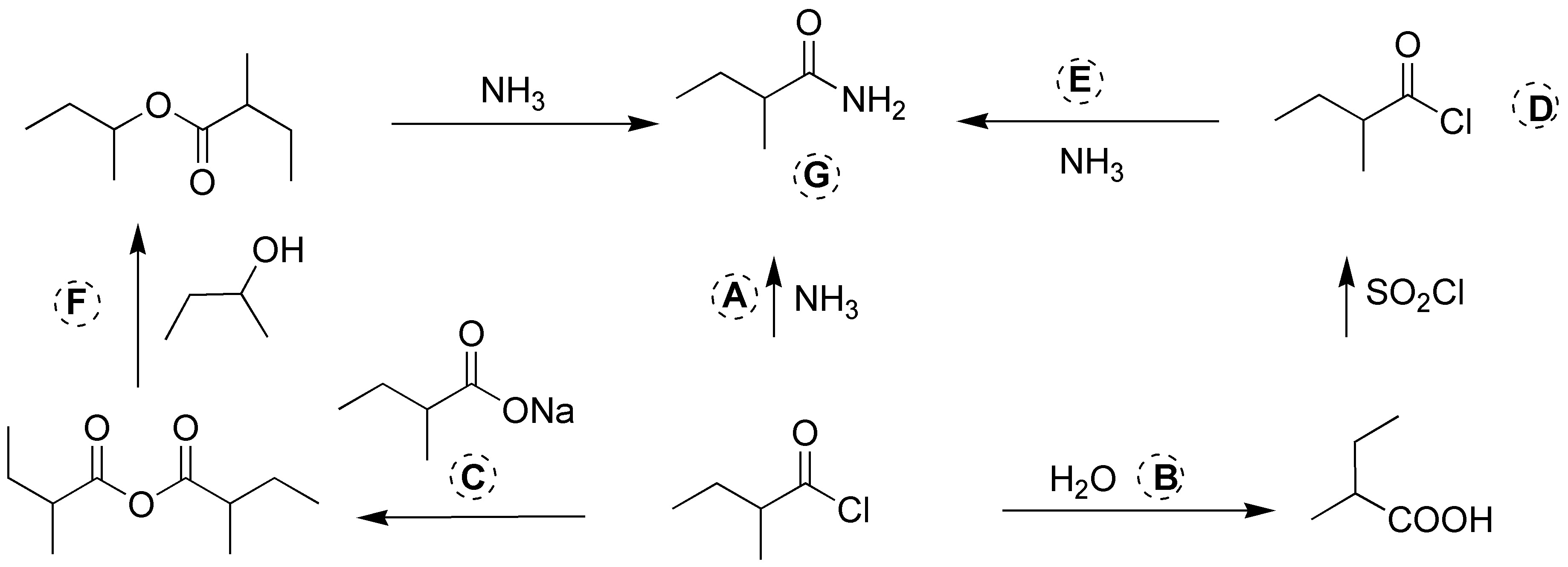
Solution 17:
This is an imide-forming reaction from a carboxylic acid, in this case the two acid functional groups are attached to an aromatic ring. Ammonia, through an acid-base reaction, reacts with the carboxylic acids to give the corresponding salt (2 moles of NH3 are needed to react with the two acid groups). This salt on heating forms the imide (analogous to the acid anhydride but instead of having an oxygen between the carbonyl groups it presents an NH), giving off two molecules of water and one molecule of ammonia.
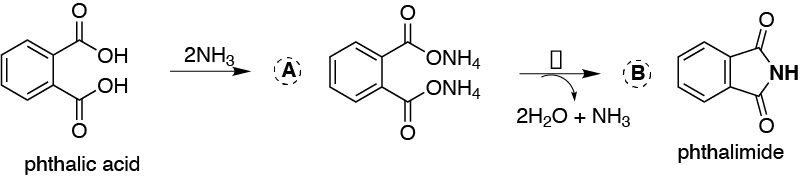
Solution 18:
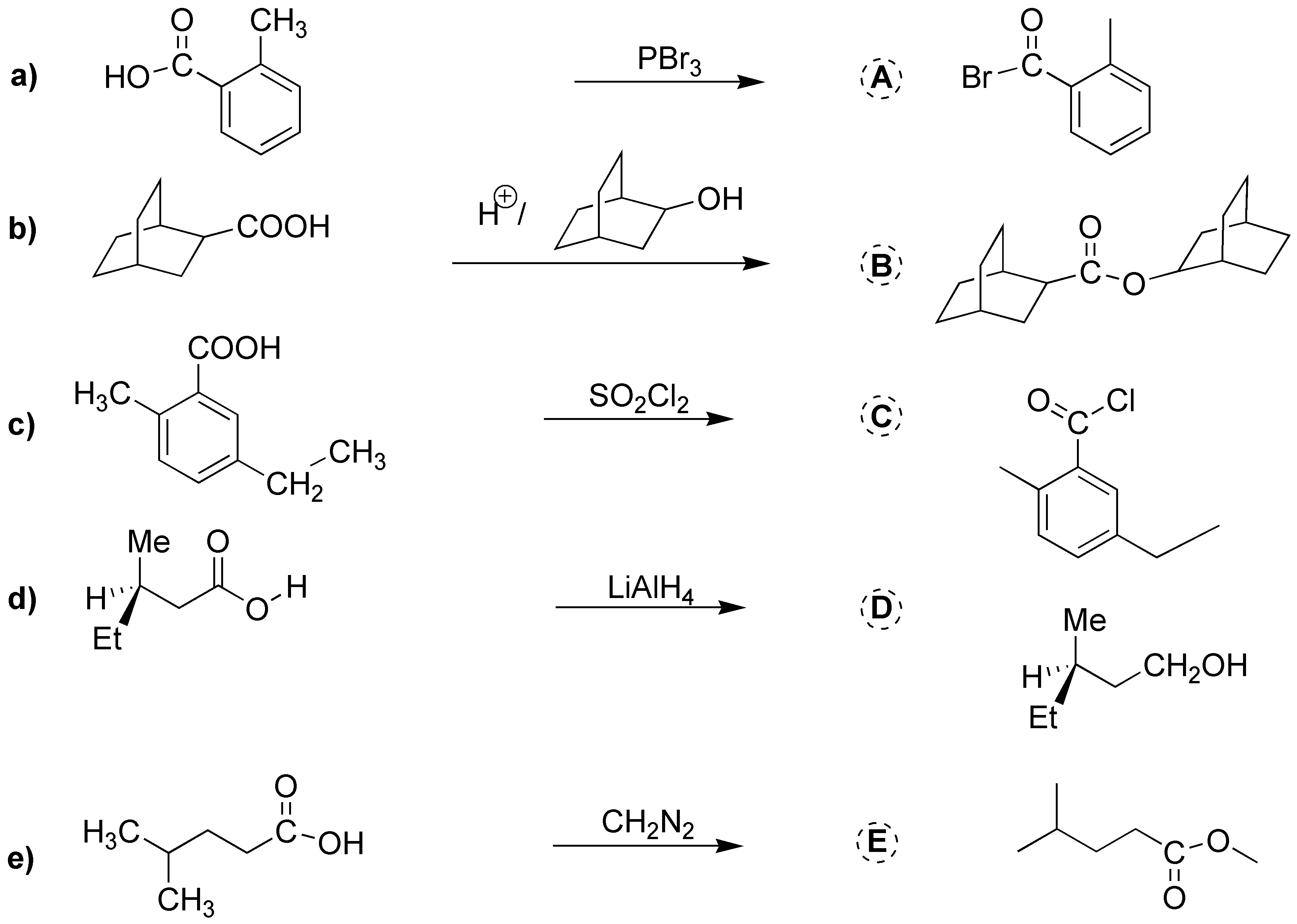
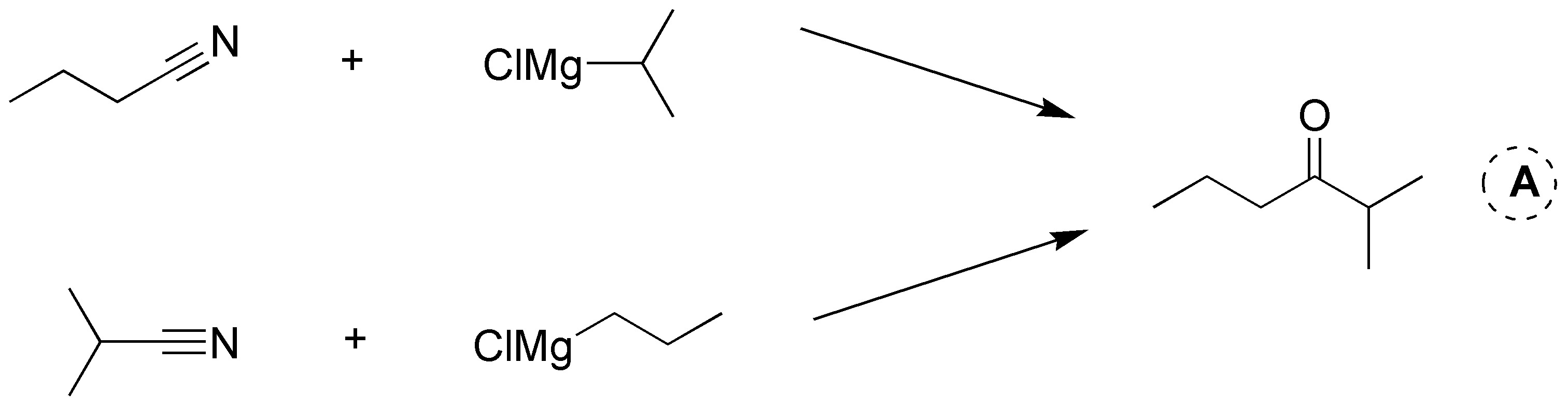
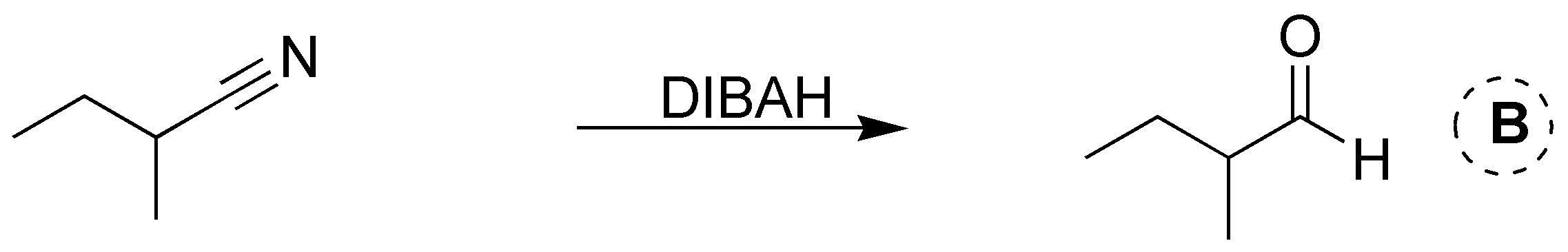
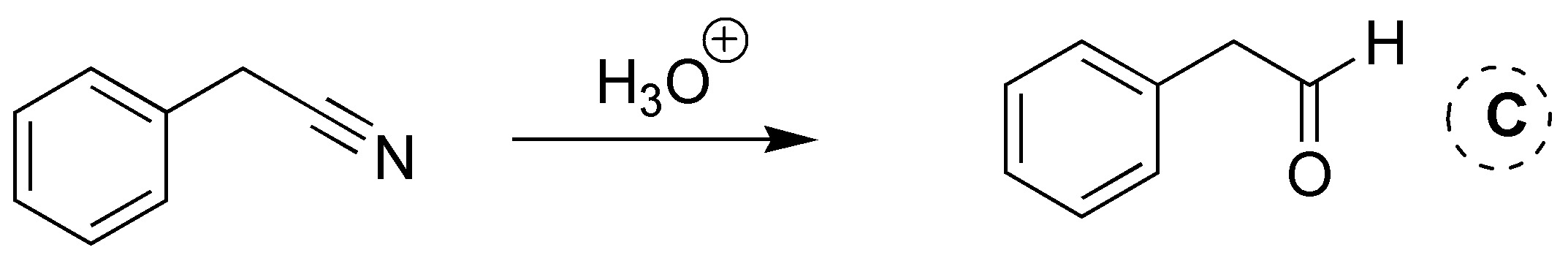
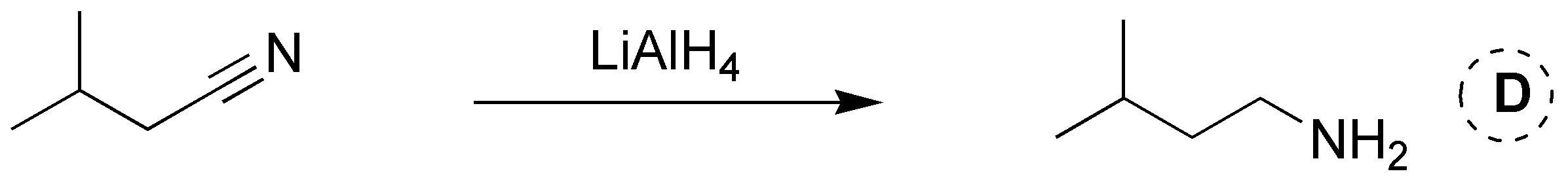
Solution 19:
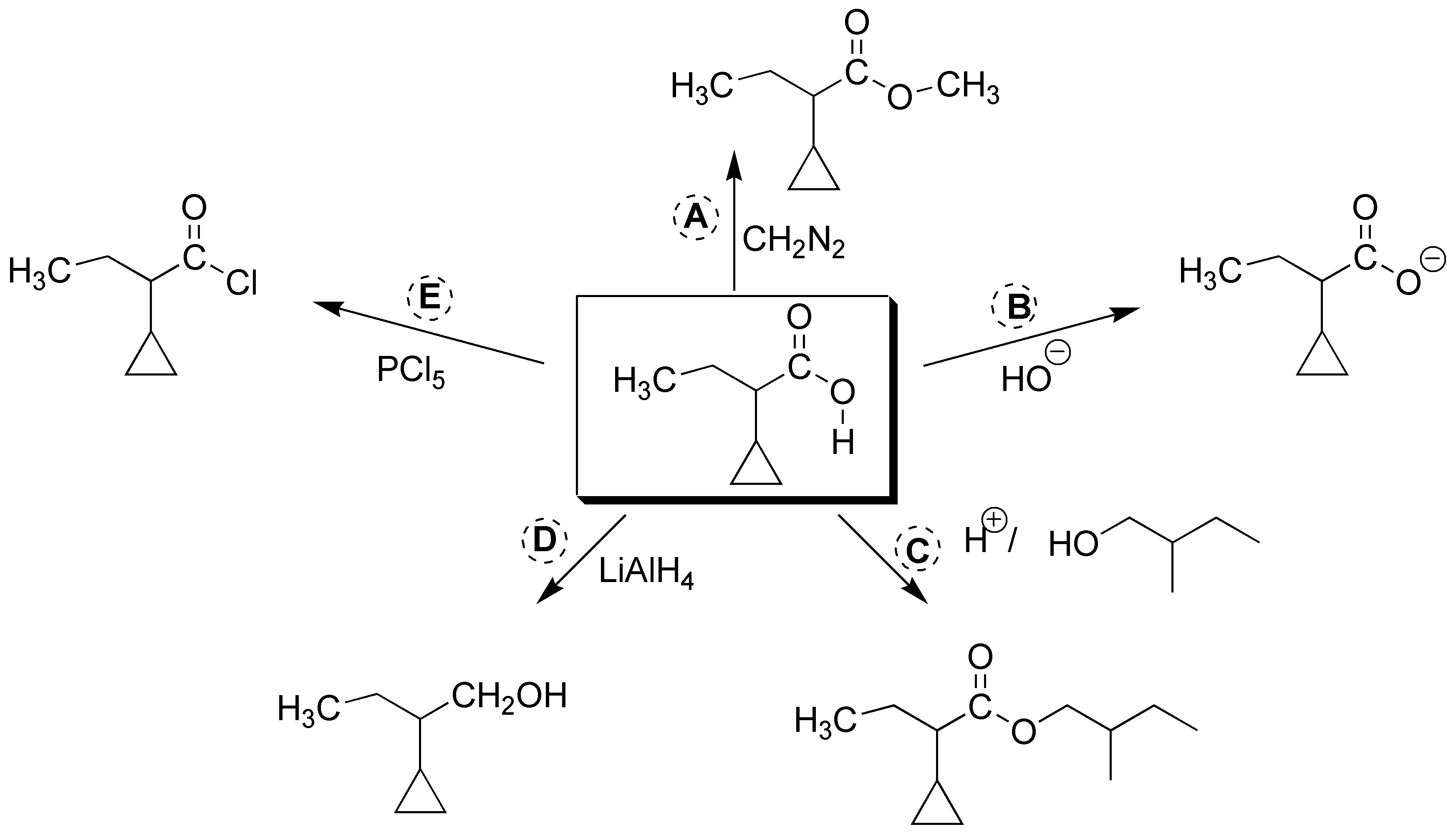
Solution 20:
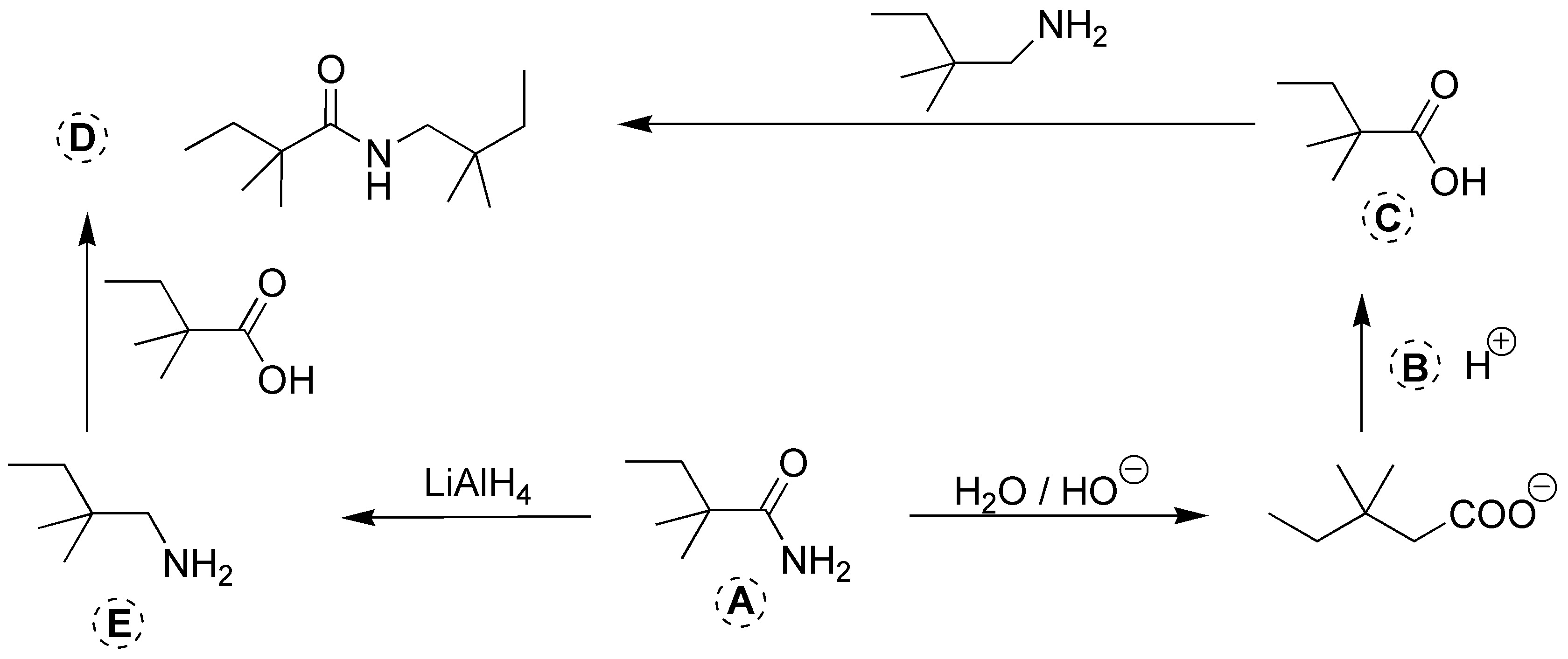
Solution 21:
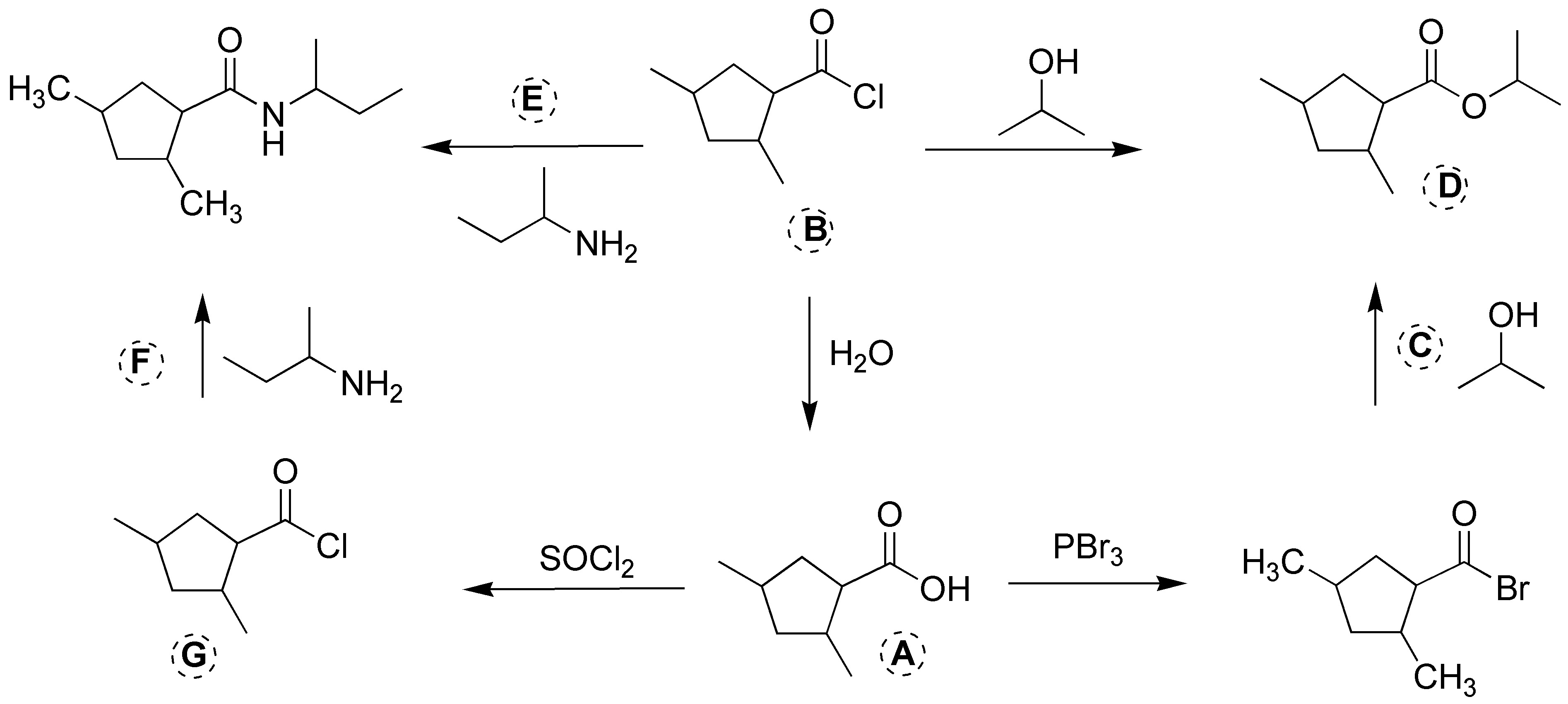
Solution 22:
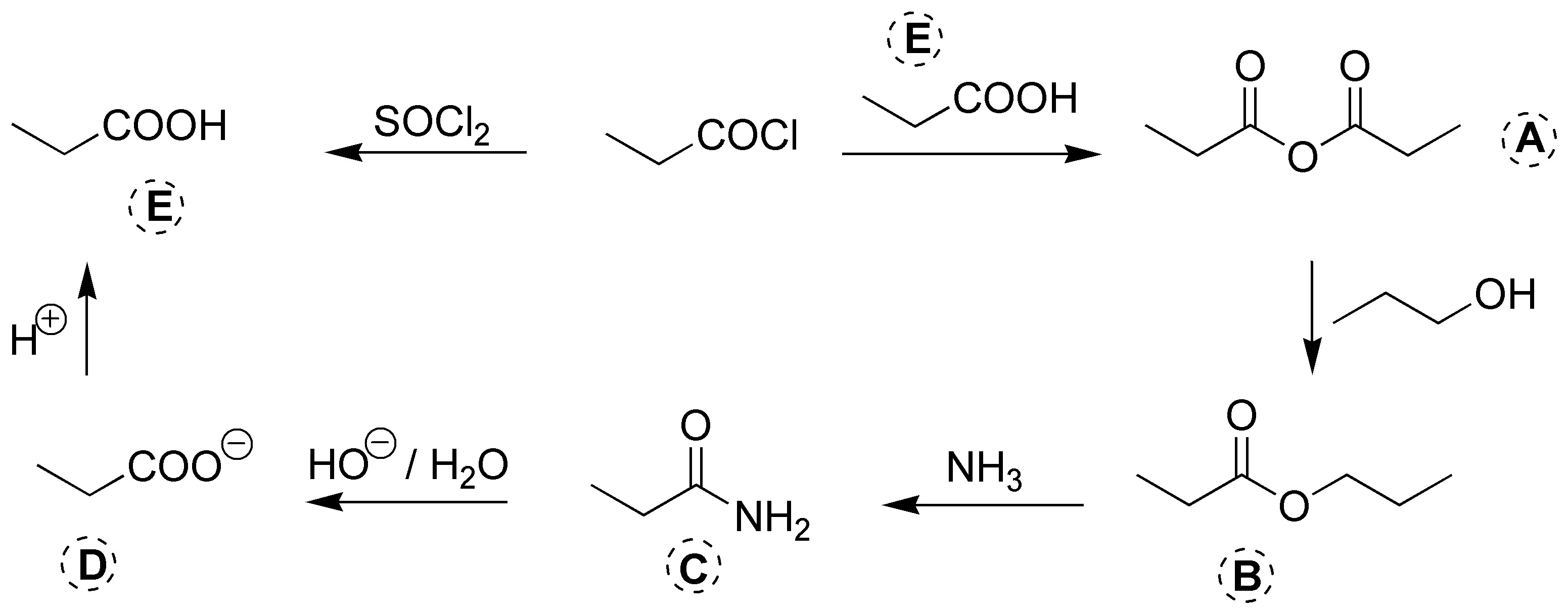
Solution 23:
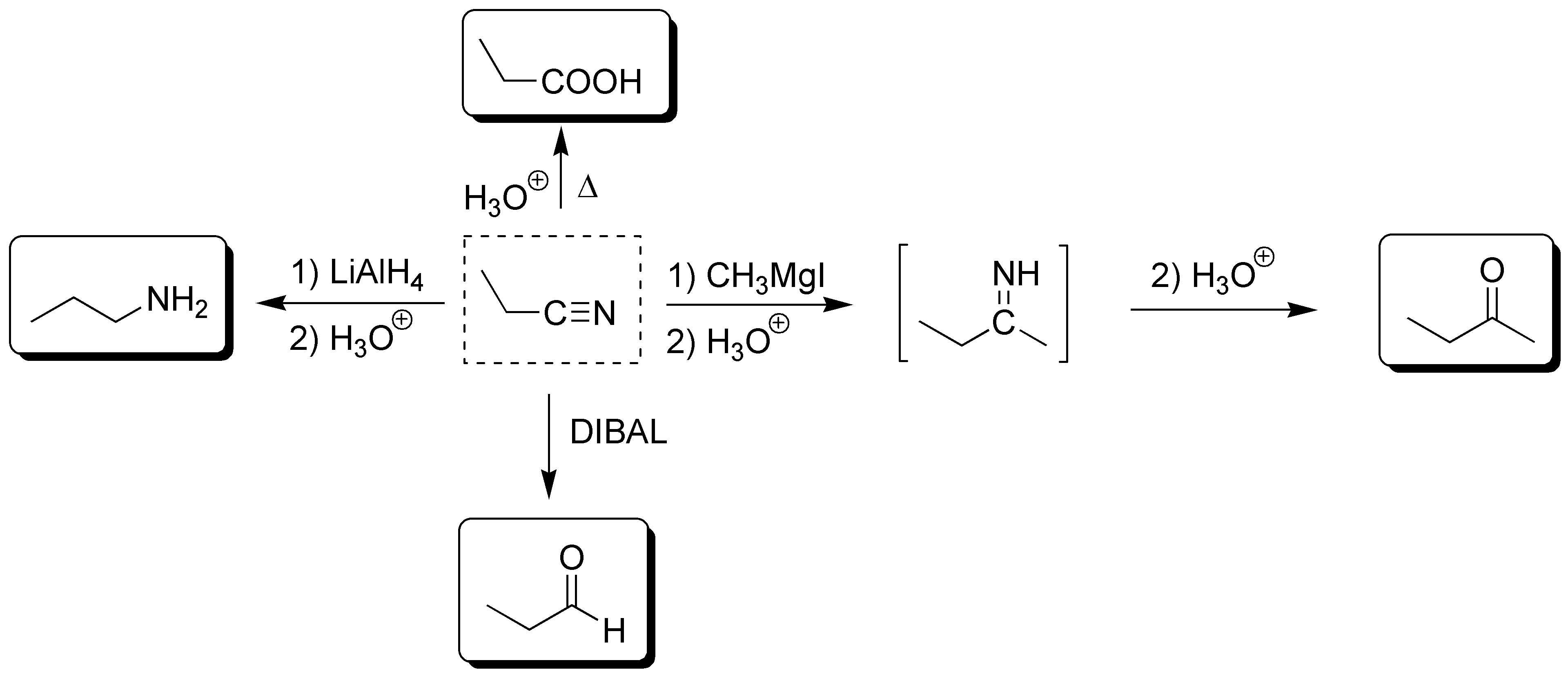
Solution 24:
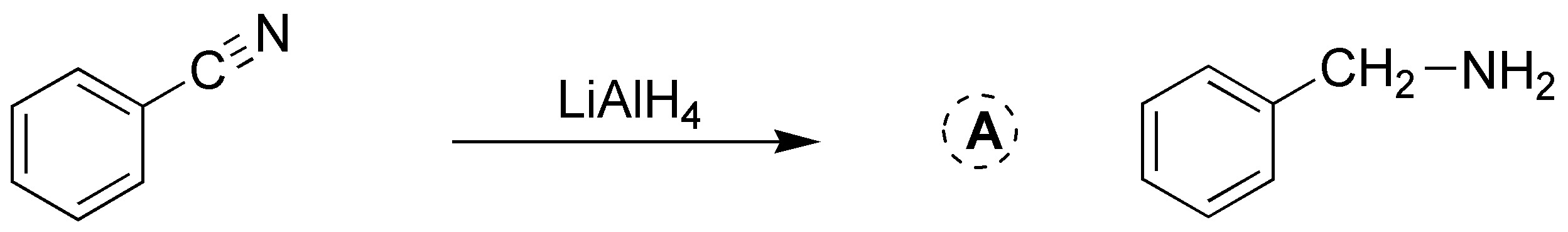
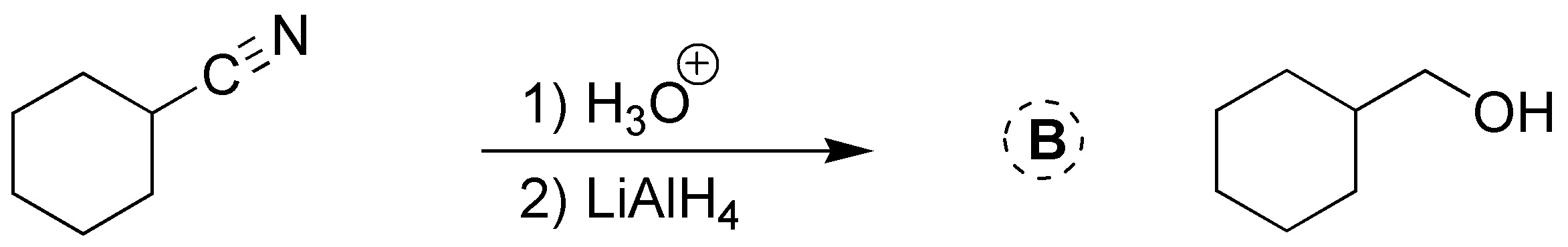
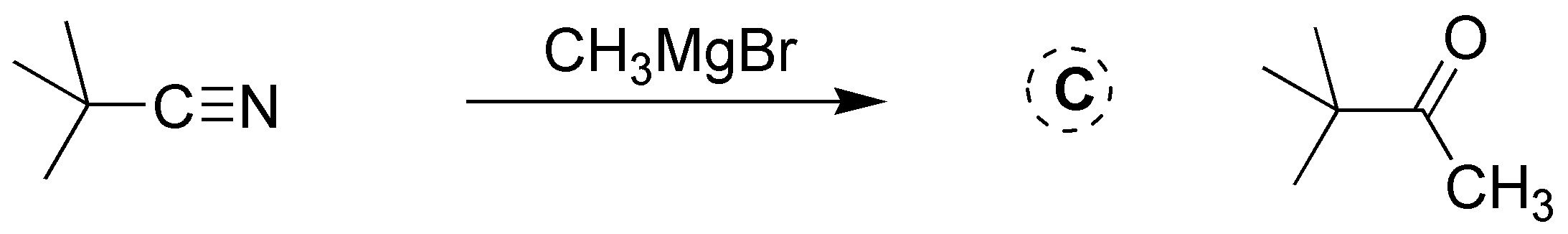
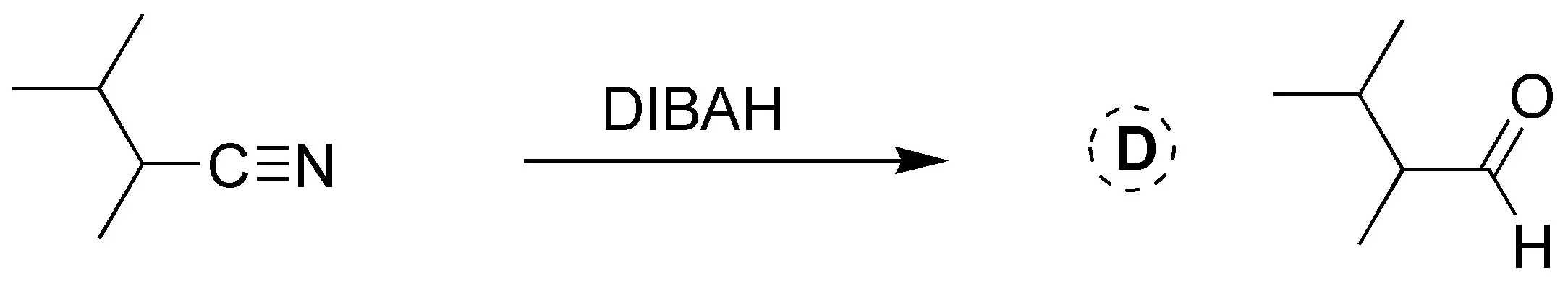
Solution 25:
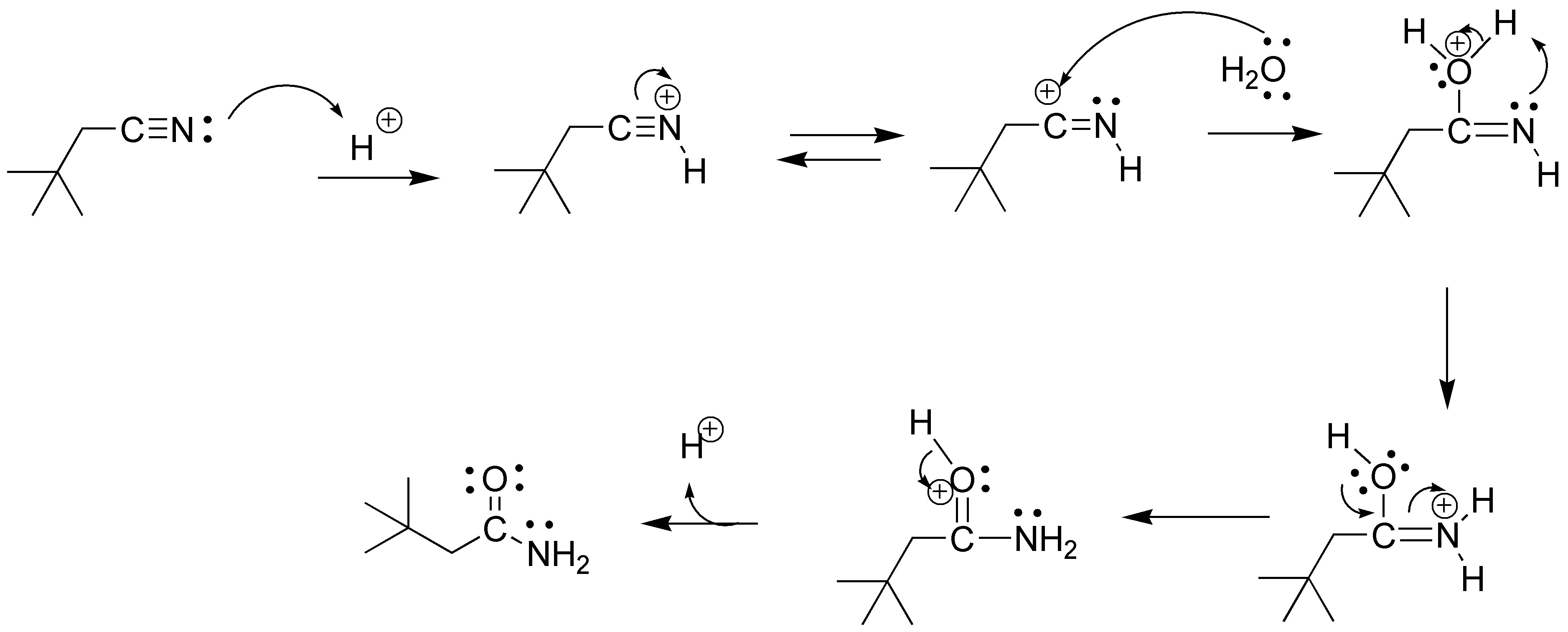
Solution 26: