Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Haloalkanes – Alkyl halides – solutions to the problems
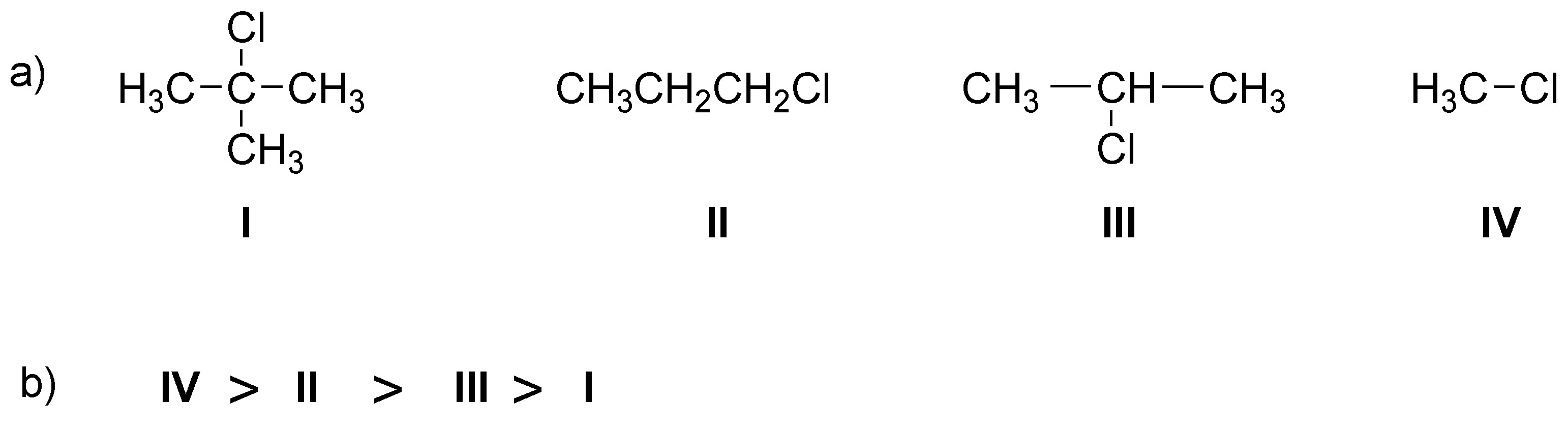
Solution 1:
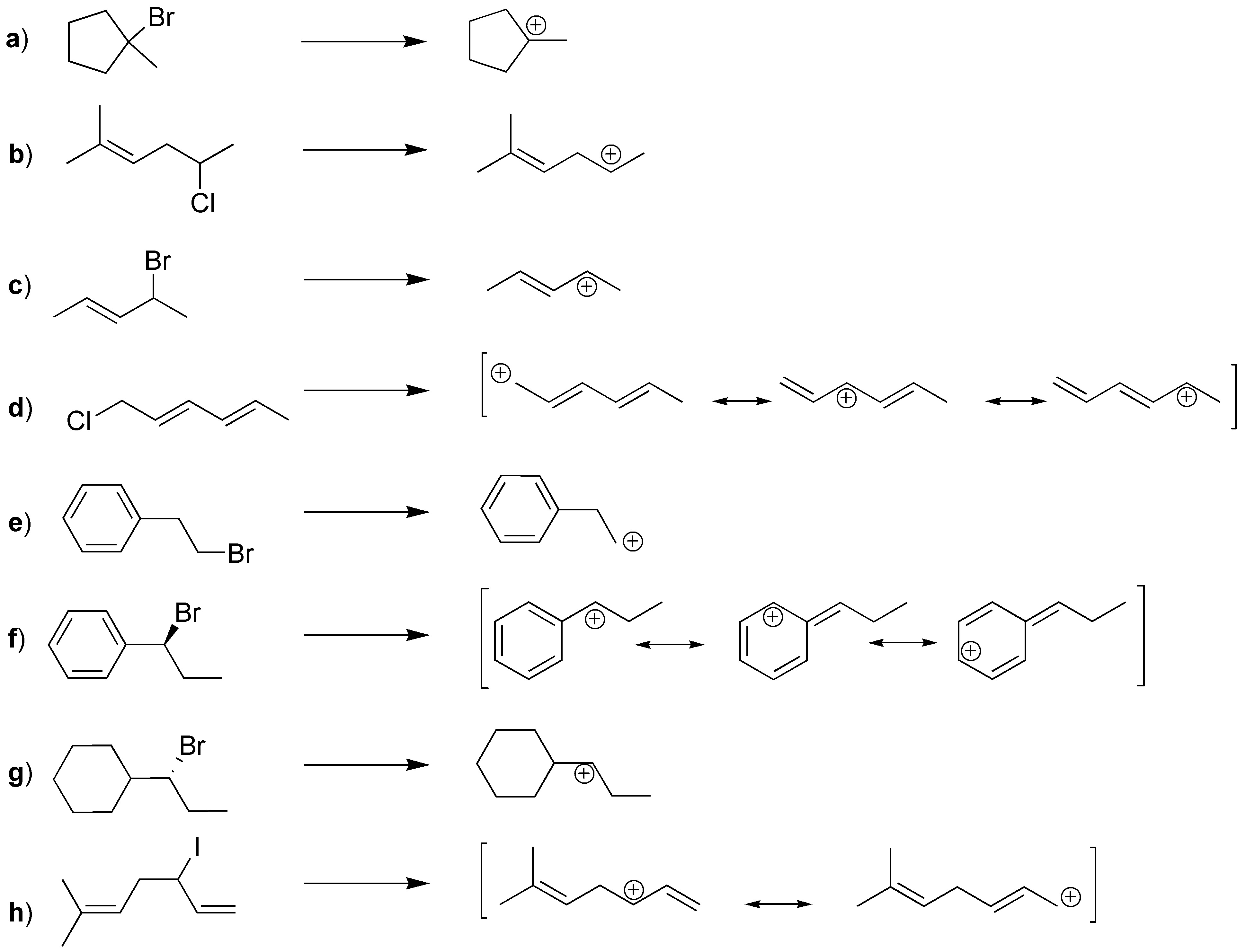
(a) is a tertiary bromide, (b) is a secondary chloride, (c) is a secondary and allylic bromide, (d) is a primary and allylic chloride, (e) is a primary bromide, (f) is a secondary and benzyl bromide, (g) is a secondary bromide, and (h) is a secondary and allylic iodide. The carbocations obtained for each of them are given below. In addition, for d), f) and h) more than one carbocation is obtained by resonance.
Solution 2
(a) secondary; (b) benzylic; (c) vinylic; (d) tertiary; (e) allylic; (f) primary.
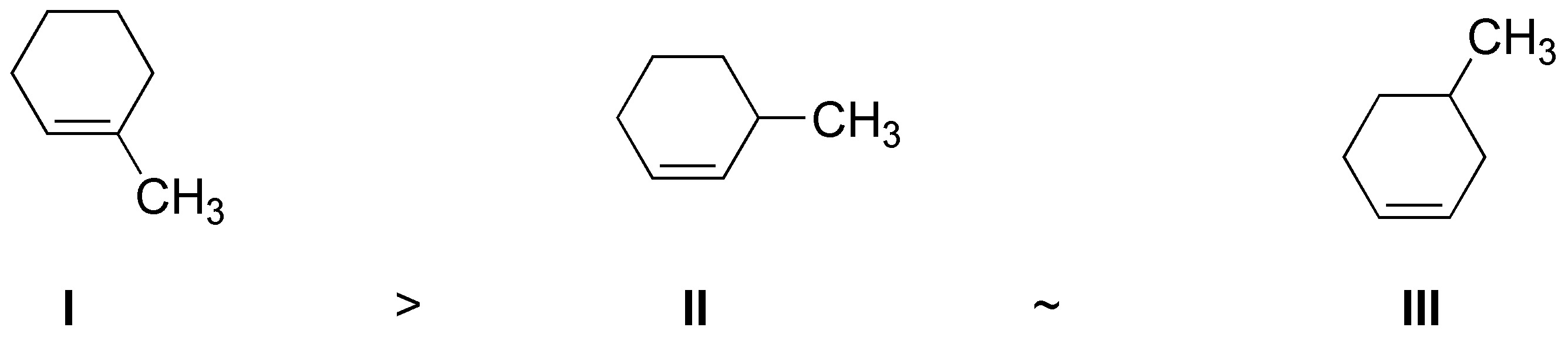
Solution 3:
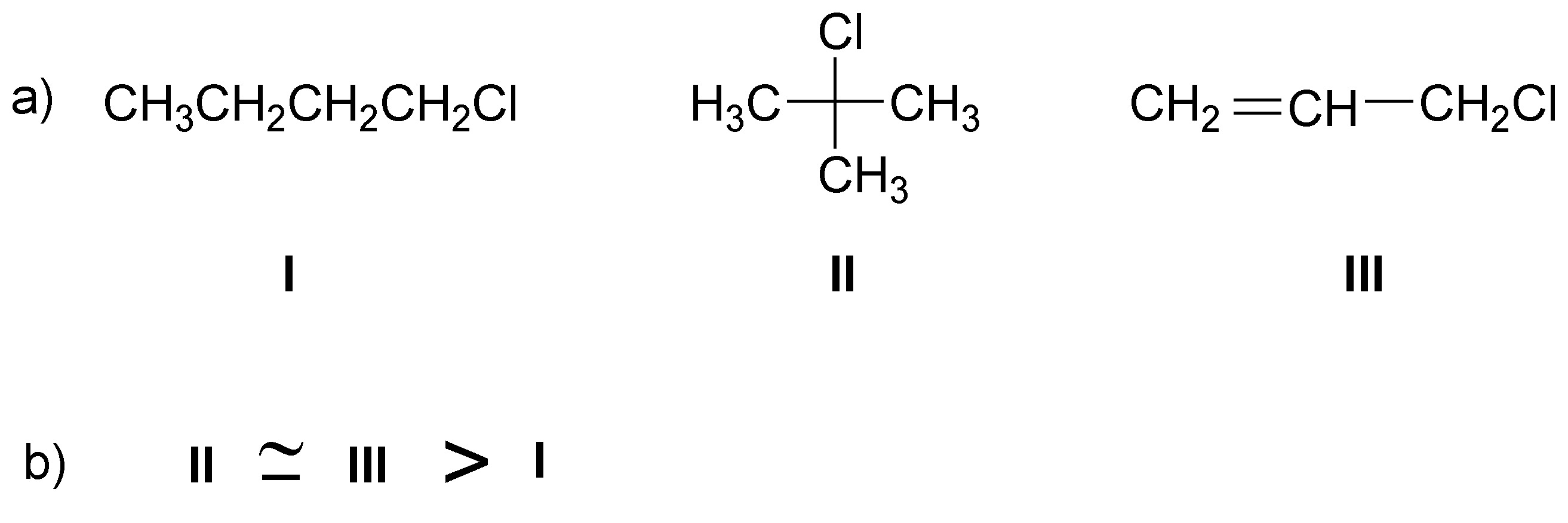
SN1 involves the formation of a carbocation so as II and III give rise to very stable carbocations (allylic and tertiary) with respect to I (primary) both will react faster. The structures will be:
Solution 4:
The reactivity in SN2 is given by steric hindrance so the order will be: methyl, primary, secondary and tertiary.
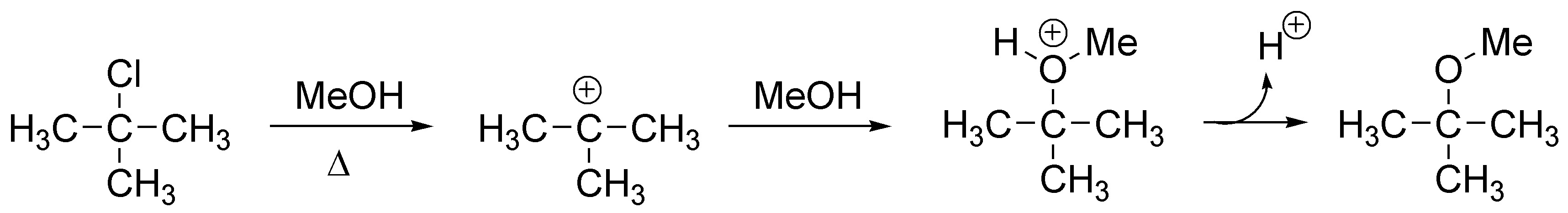
Solution 5:
Since it is a tertiary chloroalkane the initial stage will be the formation of a carbocation (loss of chloride ion). This carbocation is attacked by methanol (nucleophile) generating an alkoxonium ion which by loss of a proton generates the corresponding tert-butylmethyl ether:
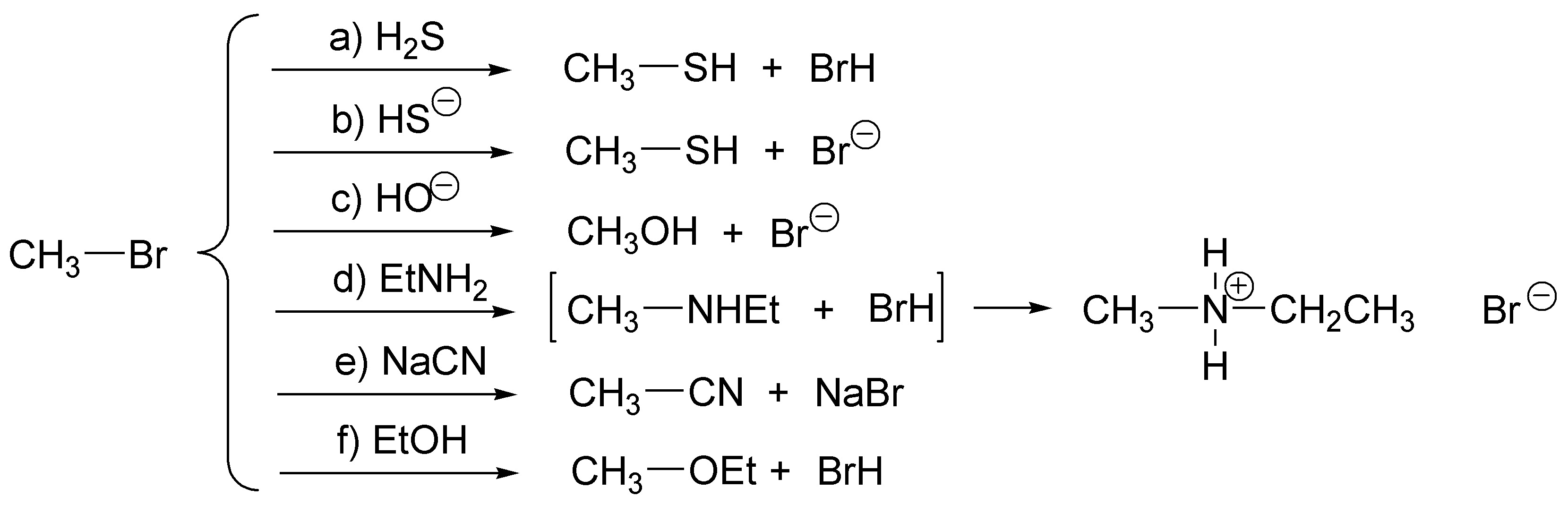
Solution 6:
Being a methyl halide it would be bimolecular nucleophilic substitution reactions:
Solution 7:
Being a primary halide it would be bimolecular nucleophilic substitution reactions and the reagents needed would be the following nucleophiles:
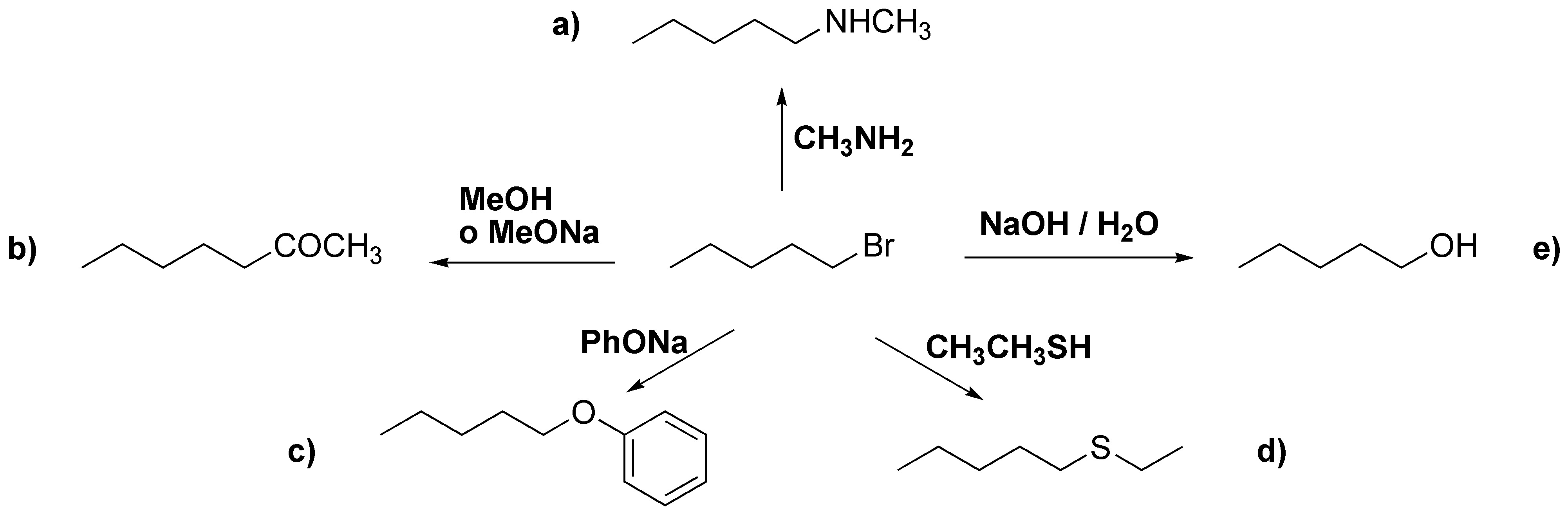
(a) methylamine; (b) methanol or sodium methoxide; (c) sodium phenolate; (d) ethylmercaptan (ethanethiol); (e) sodium hydroxide in water.
Solution 8:
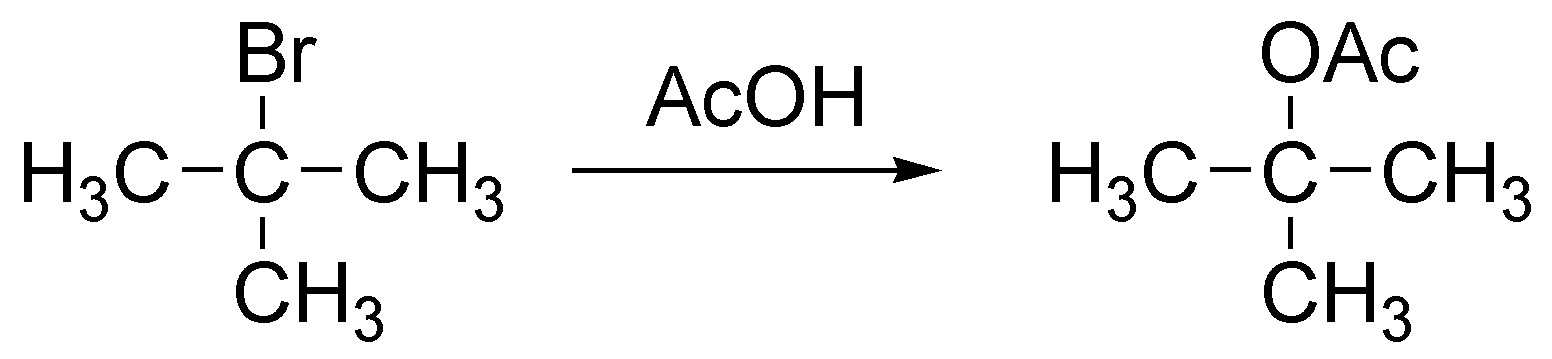
(a) It is an SN1 reaction because it is a tertiary haloalkane.
(b) The rate-determining step is the formation of the tertiary carbocation.
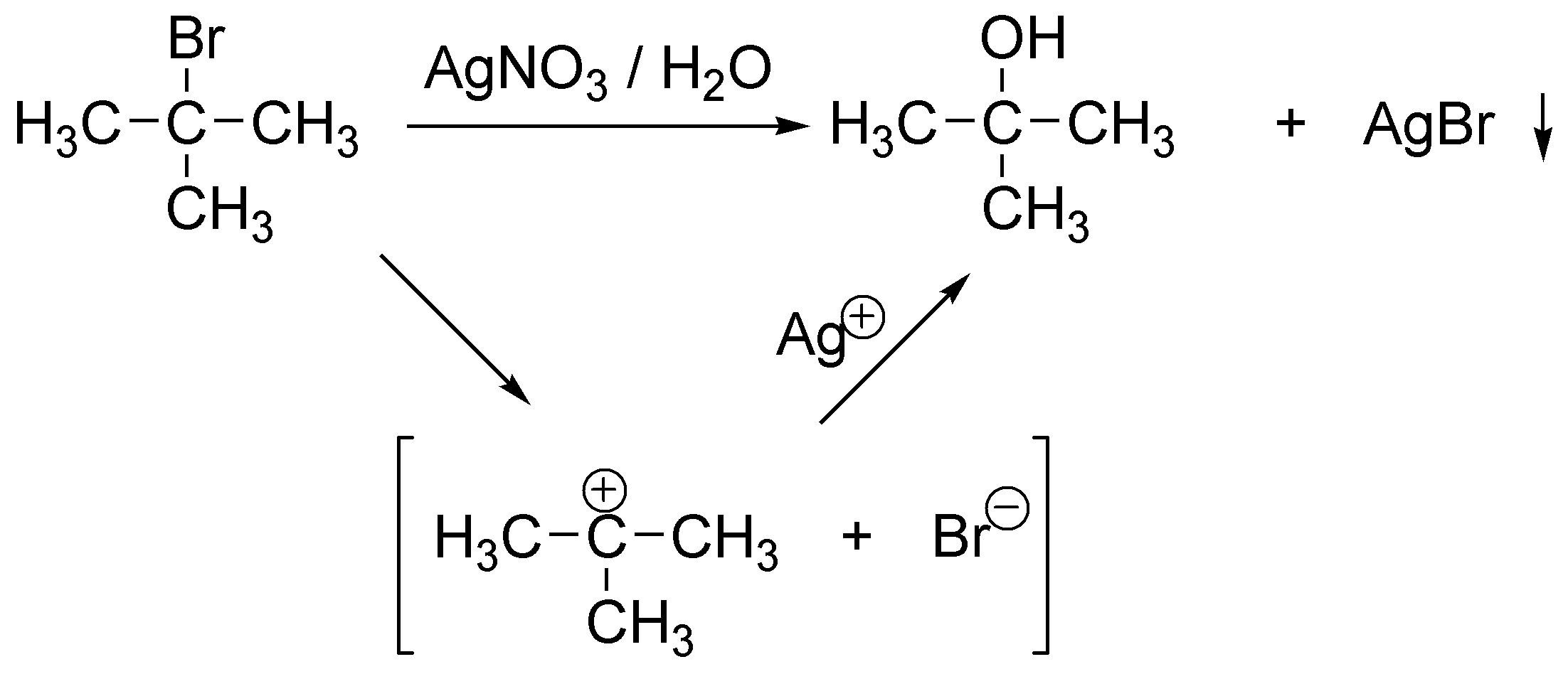
Solution 9:
It is an SN1.
Solution 10:
This is an SN2 reaction and therefore the relative reactivities will depend on the degree of substitution of the substrate: in the first and third cases it is a question of primary versus secondary bromides and in the second case it is a question of which is the better leaving group (bromide versus chloride).
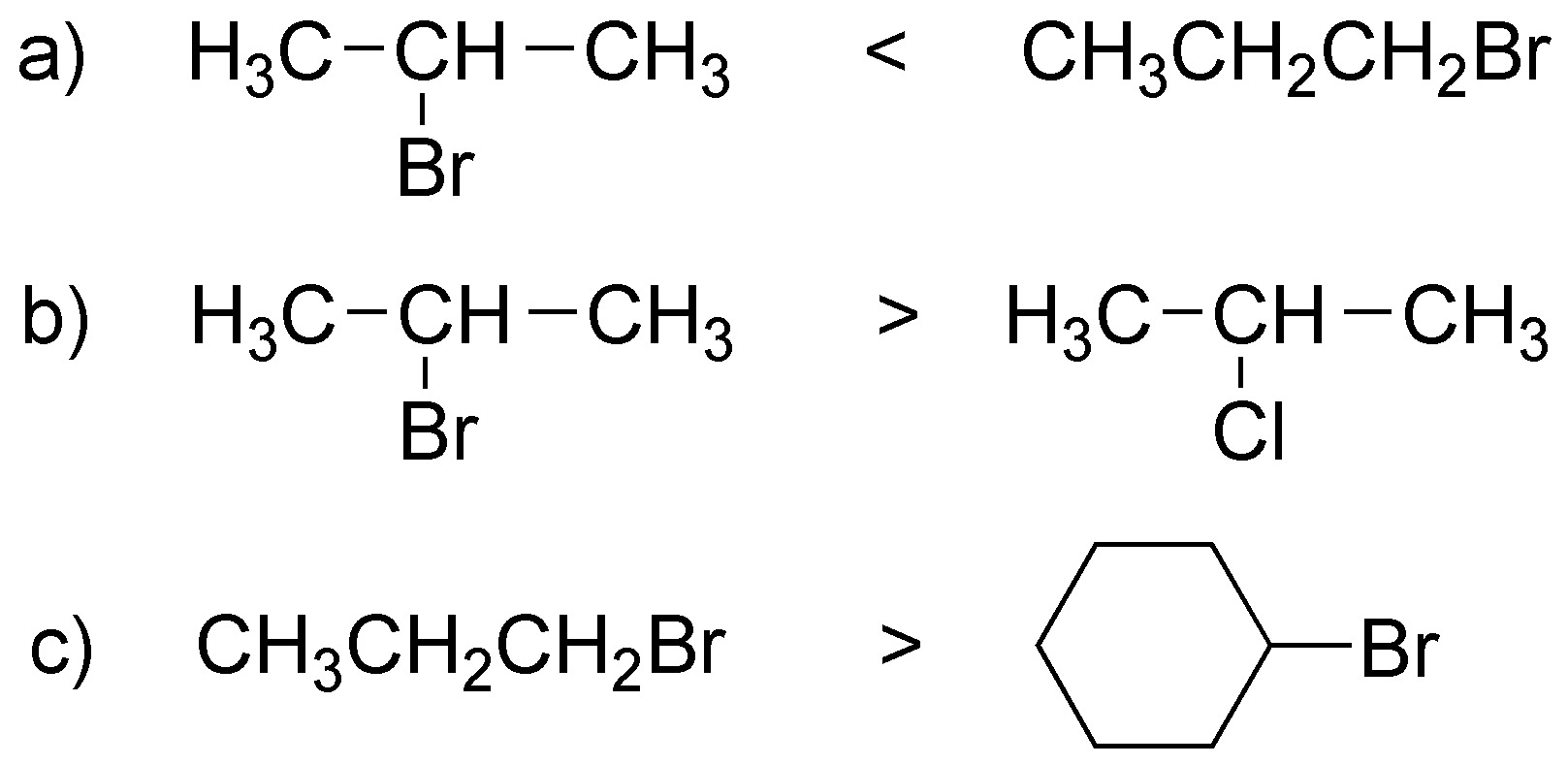
Solution 11:
This is an SN2 reaction and therefore the relative reactivities will depend on the degree of substrate substitution; although both are primary halides the second has more steric hindrance and therefore will react slower and in worse yield (will give more E2 elimination).
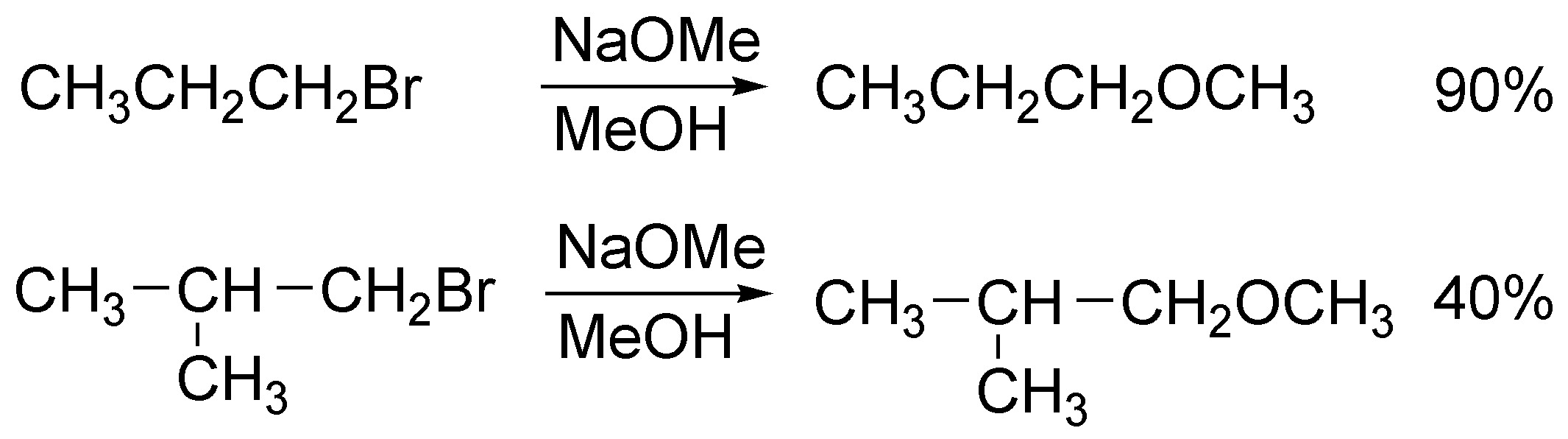
Solution 12:
It is a second order process so the reaction rate will depend on the concentrations of cyanide 1-iodopropane, v = k [R-I]·[CN–]. In the transition state the simultaneous breaking and formation of the leaving group and nucleophile bonds on the primary carbon occurs simultaneously. In this type of reaction there are no reaction intermediates, so the reaction profile is characterized by an energy maximum (transition state) as we move along the reaction coordinate from reactants to products. If the cyanide concentration is decreased and the iodide concentration is maintained, the reaction rate is halved.
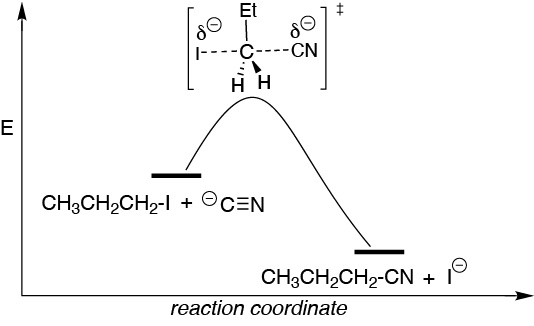
Solution 13:
(a) I– ion is more nucleophilic than Cl–. Nucleophilicity increases as one moves down a group in the periodic table.
b) Sulfur is more nucleophilic than oxygen for the same reason as in the previous section.
c) Water is a relatively weak nucleophile compared to the hydroxyl ion because the electron density is higher on the oxygen atom for hydroxyl than for water.
d) Methoxide is more nucleophilic than fluoride. Other things being equal, the nucleophilicity of two atoms in the same row of the periodic table increases to the left. In addition, the pKa values for conjugated acids are 5 for HF and 16 for CH3OH. The lower the pKa value indicates that the acid is stronger and therefore, its conjugate base will be weaker.
Solution 14:
(a) Reactions I (NaOH/MeOH), II (NaOH/DMSO), and V (H2O)lead to the formation of 2-methylbutan-1-ol. Reactions III (NaSH/MeOH) and IV (NaSH/DMSO) produce 2-methyl-butanethiol. In all cases the mechanism of the reactions is SN2 type.
![]()
b) The fastest reaction will be the one in which the strongest nucleophile is used with an aprotic solvent. These conditions occur in the case of reactions IV and II.
Solution 15:
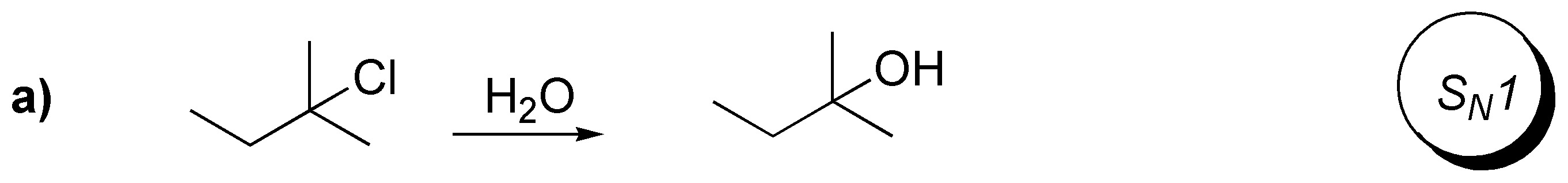
a) Given the nature of the substrate, which is a tertiary halide, and of the reactant, which is a weak, polar, aprotic nucleophile, the most likely mechanism is an SN1 type reaction, and in this case it is a solvolysis reaction.
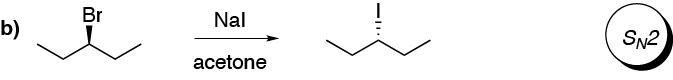
b) The reaction is of type SN2, since it takes place on a secondary substrate with inversion of the configuration and using a good nucleophile and an aprotic polar solvent such as acetone.
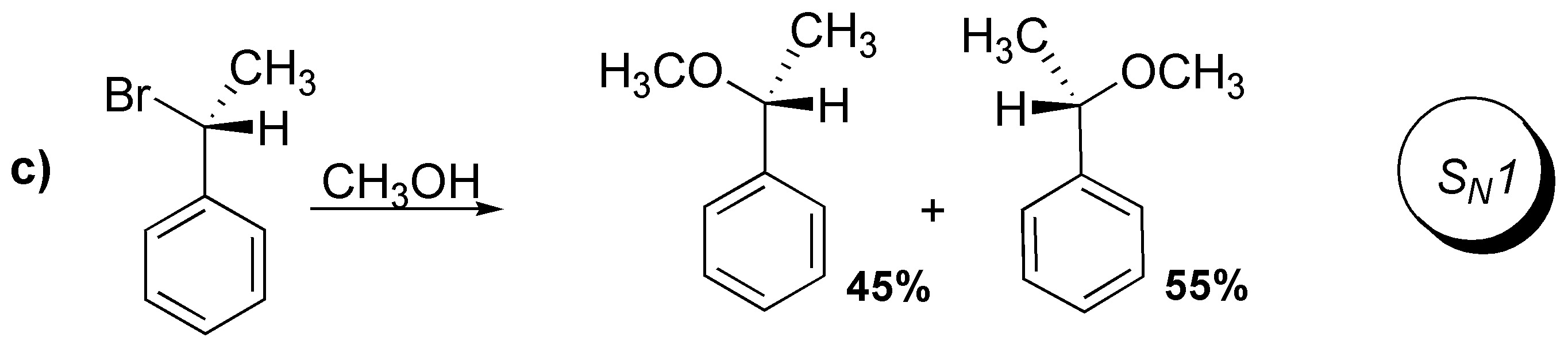
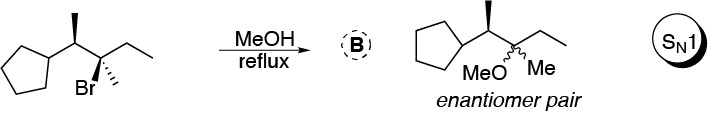
c) It starts from a chiral substrate and results in a mixture of two enantiomers in similar proportions. The starting halide is secondary, and the carbocation generated is secondary and benzylic. The nucleophile (methanol) attacks on both sides of the carbocation without marked preference, so the reaction type is SN1.
d) The reaction type is SN2 due to the reaction conditions. It is a substrate similar to the previous case and the reaction is carried out in the presence of a good nucleophile such as cyanide and with an aprotic polar solvent such as DMSO. Therefore, configuration inversion occurs.
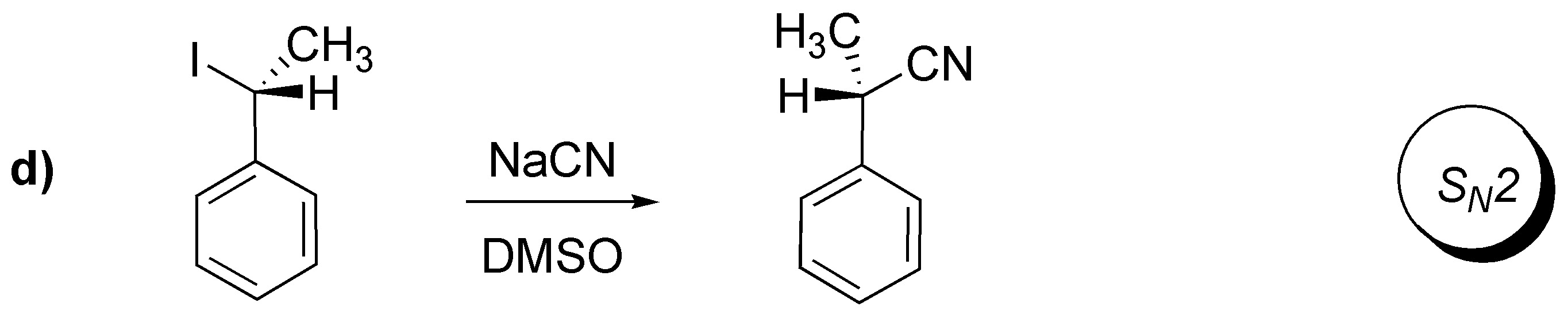
Solution 16:
A.- A bromine atom is substituted by an OH group with configuration inversion. To carry out this transformation the suitable reagent is NaOH.
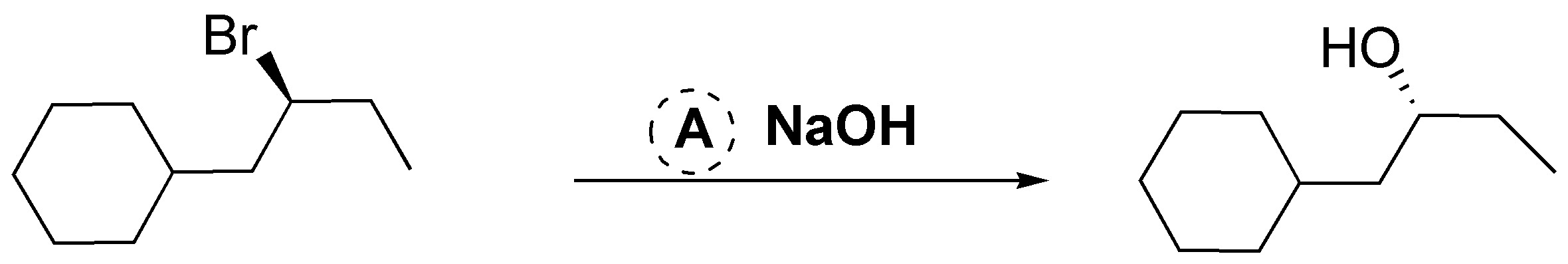
B.- In this case there will be no reaction, since the bromine is in a primary position, so a solvolysis reaction is very improbable, and water is not sufficiently nucleophilic to give the SN2 reaction. On the other hand, chlorine on the benzene ring is inert under these conditions.
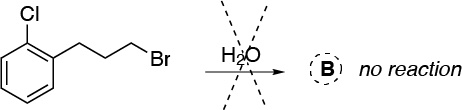
C.- Primary and secondary allyl halides react rapidly with nucleophiles by an SN2 mechanism. Thiols easily form with bases the corresponding thiolates, which are excellent nucleophiles so the following product will be obtained with inversion of the configuration:
D.- This is a solvolysis with methanol. The reaction intermediate is a secondary and allylic carbocation that is stabilized by resonance, so that the nucleophile can attack in the two positions where there is greater positive charge density. At one of the positions the racemic mixture of products would be obtained.
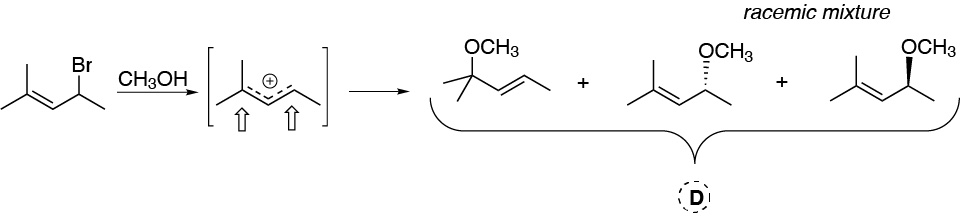
Solution 17:
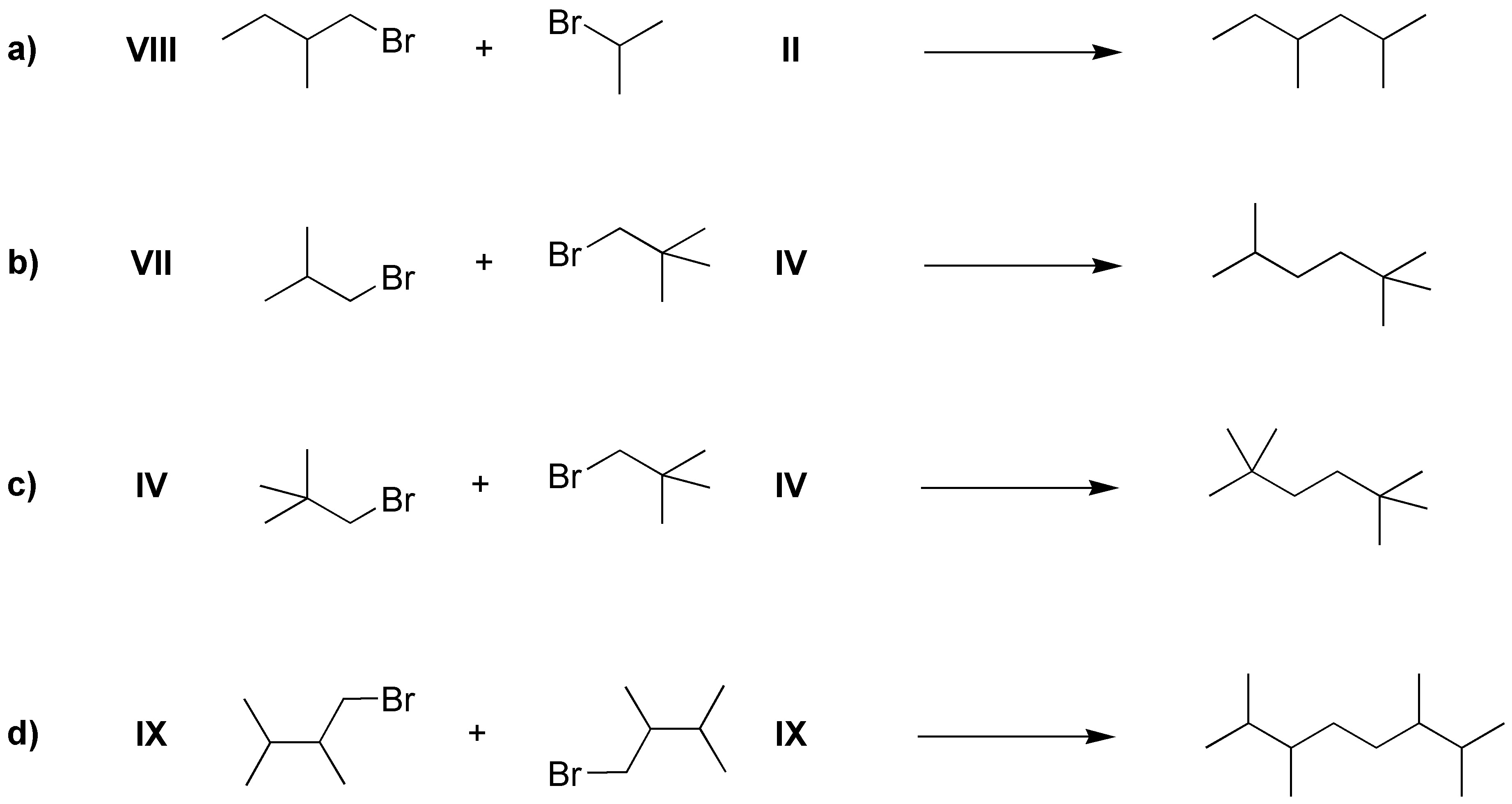
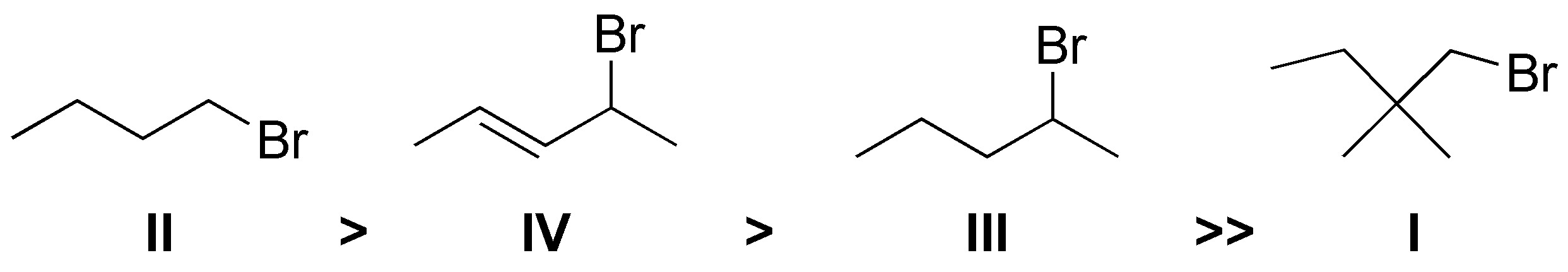
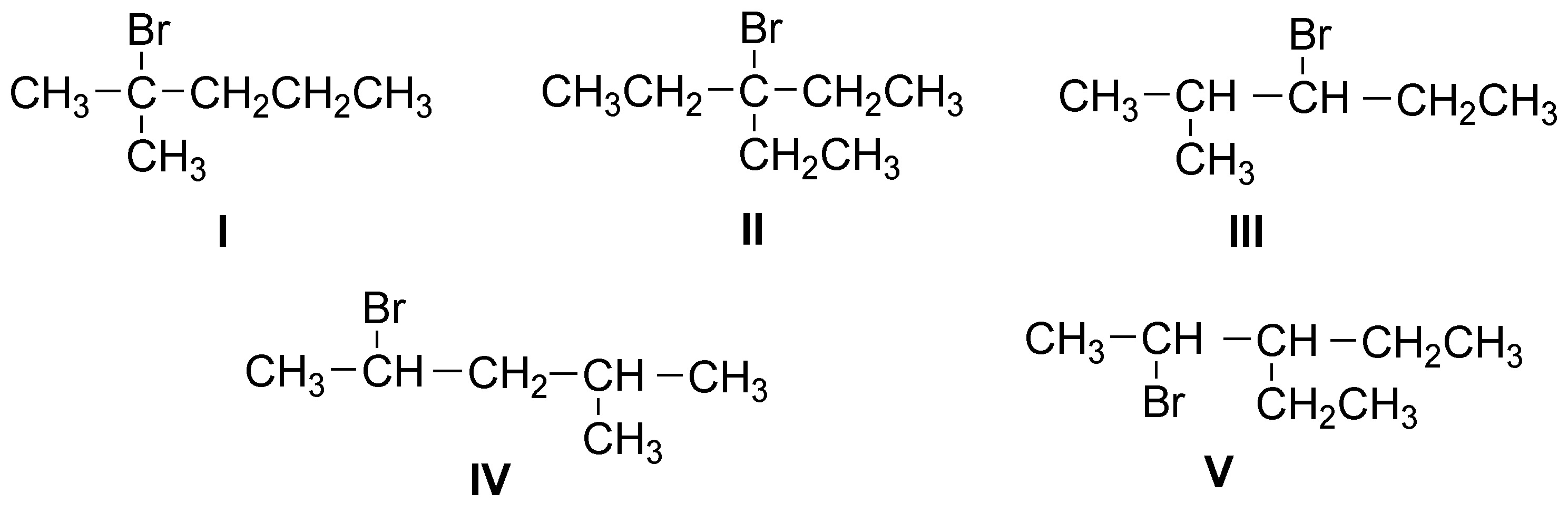
The order of reactivity is II > IV > III >> I. The substrate that presents the greatest facility for SN2 is II, since it is a primary bromide with low steric hindrance to the approach of a nucleophile. In decreasing order of reaction rate it is followed by substrate IV as it is secondary and also allylic. Next would be III since it is secondary and lastly I which would be practically inert since the substituents in the β-position impede the approach of the nucleophile.
Solution 18:
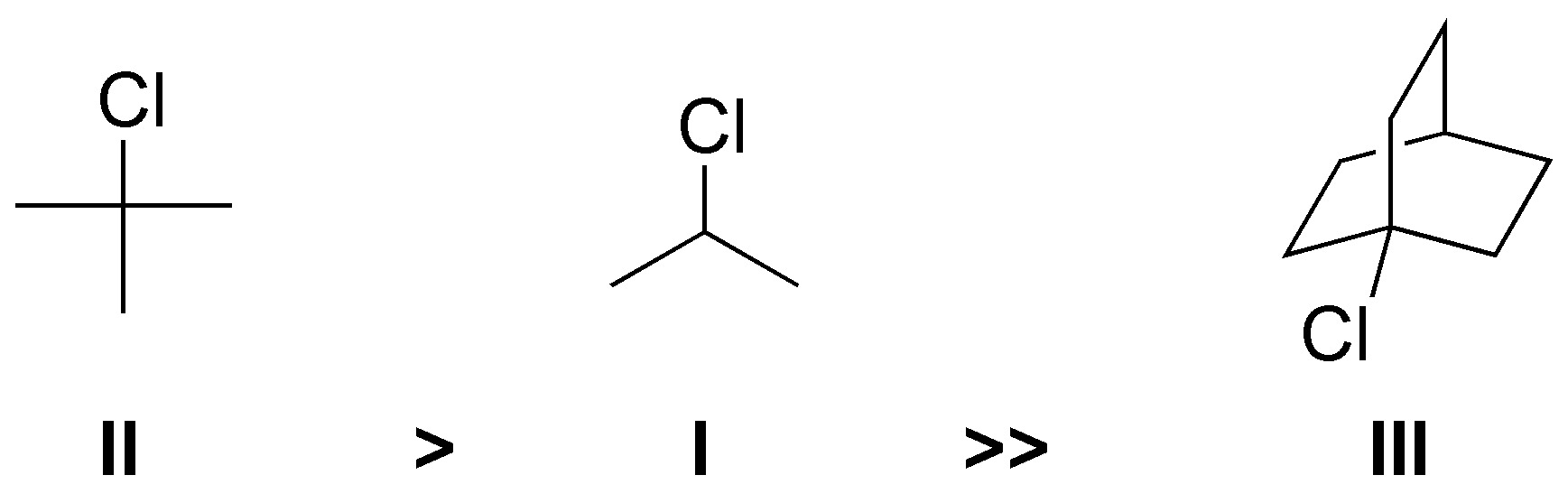
The rate of solvolysis of a substrate is given by its ability to form stable carbocations, since the reaction proceeds through an SN1 mechanism. II generates a tertiary carbocation, I a secondary one while III is a rigid structure that prevents the formation of a conventional carbocation. The order of reactivity is II > I >> III.
Solution 19:
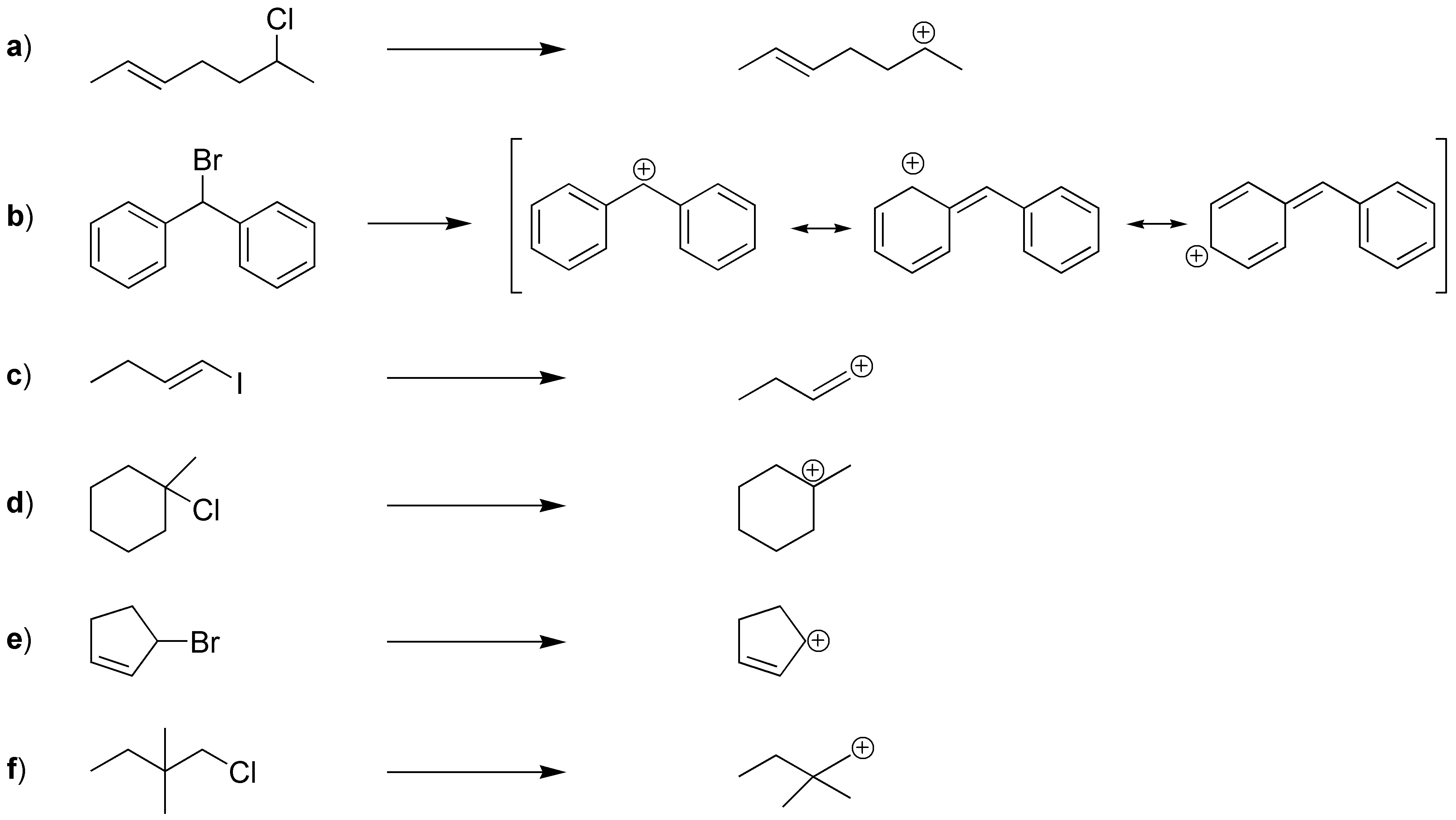
The reaction described is a solvolysis, which in the case of using water as solvent, receives the specific name of hydrolysis. The reaction proceeds by an SN1 mechanism, via a carbocation. The carbocation formed in the first instance is secondary. However, the product obtained suggests the attack of the solvent molecule in a tertiary position. To justify this fact, a transposition of a hydrogen atom as a hydride must occur according to the following scheme:
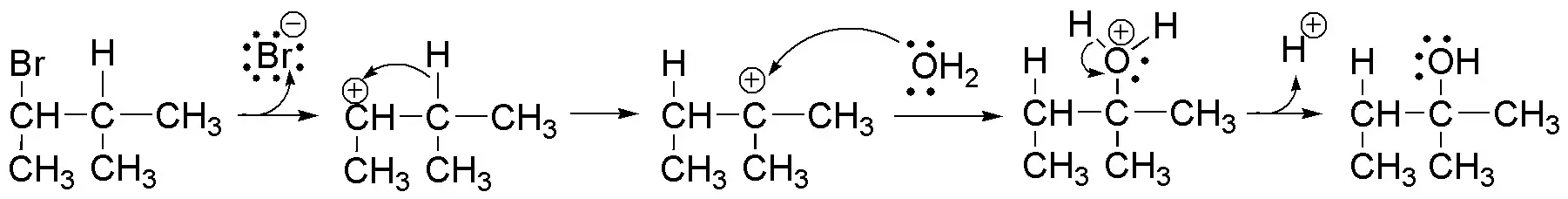
The driving force of the transformation is the formation of a tertiary carbocation, much more stable than the secondary one.
Solution 20:
The reaction is of the intramolecular SN2 type, as the base generates an alkoxide which acts as a nucleophile and displaces the chlorine found in the molecule itself resulting in the formation of a five-membered cycle
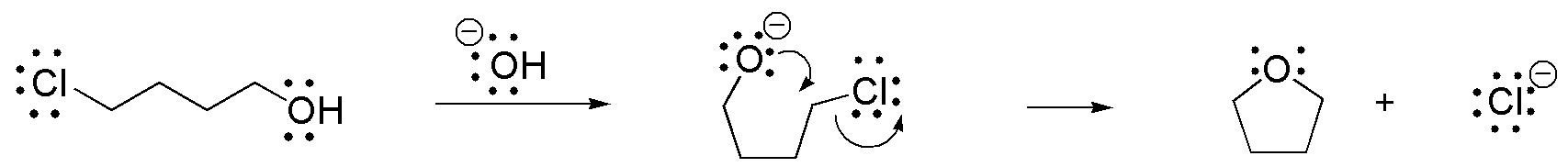
Solution 21:
(a) In the figure below, the hydrogens in the α-position are indicated by a dashed circle:
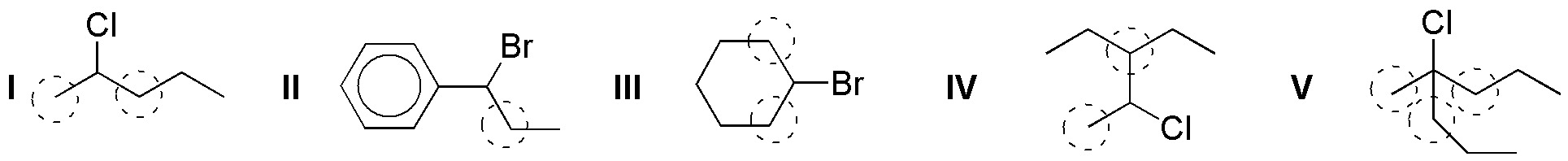
(b) Under the right conditions elimination reactions will occur on these substrates by loss of the leaving group Cl and Br and of a hydrogen located in the α-positions.
If there is the possibility of formation of several alkenes, due to the fact that there may be several hydrogens a that are in the right position for the elimination reaction to take place, under the usual conditions in which these types of processes are carried out (non-bulky bases such as KOH or NaOEt), Zaitsev’s rule based on experimental observations tells us that “the majority product will be the most substituted alkene, and when the possibility exists, the formation of the alkene of relative configuration E (trans) predominates over Z (cis), because it is thermodynamically more stable“. According to these considerations, the result of the proposed reactions is as follows:
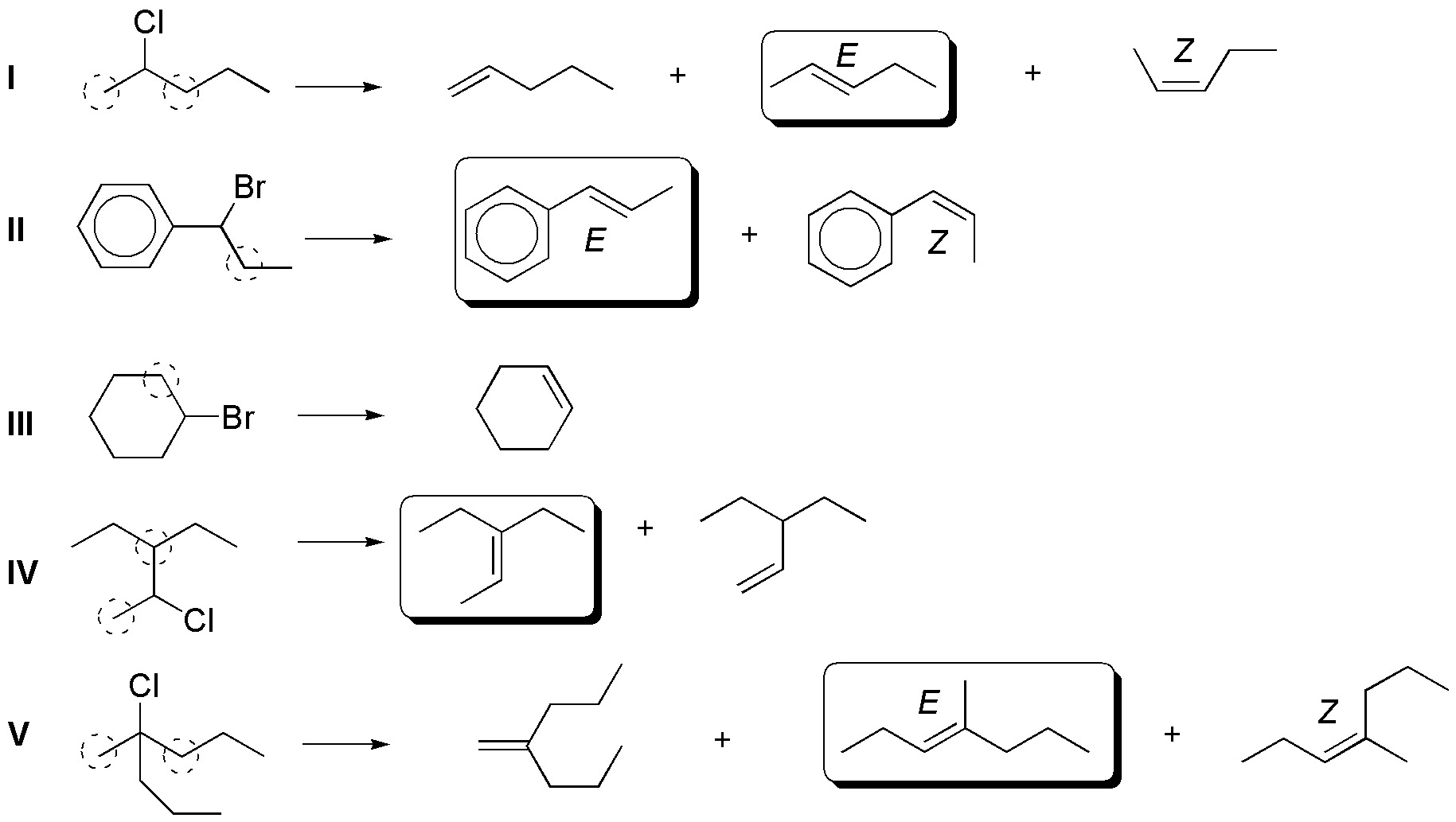
The alkenes obtained in higher proportions in I, II, IV and V are those shown with a box. For compound IIIa single product is obtained, since the marked positions lead towards the formation of the same product.
Solution 22:
Since we are dealing with primary haloalkanes, reactions a) and c) will be two SN2. While b) being a benzyl haloalkane will be a SN1.



Solution 23:
The difference between carrying out the reaction in methanol or in acetone lies in the nature of the solvent (protic in the first case and aprotic in the second). Protic solvents favor SN1 while aprotic solvents favor SN2, so racemizations tend to predominate in methanol while configuration inversions predominate in acetone.
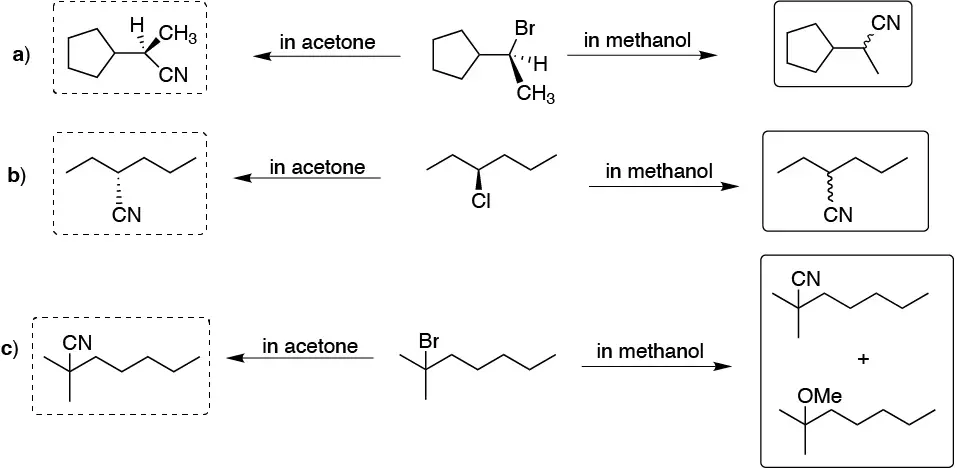
Solution 24:
(a) Being an SN1 type reaction, the concentration of methanol, which acts as solvent and nucleophile, does not influence the rate of the process.
![]()
Solution 25:
a) This is a nucleophilic substitution, the hydroxy group is transformed into a good leaving group by treatment with strong acid, becoming a hydronium ion which is substituted by the bromide ion. b) Solvolysis of a secondary bromo alkane although slow is possible. c) In the substitution of the primary halide a transposition can occur giving rise to the tertiary alcohol; however elimination would predominate. d) It is an intramolecular SN2 the thiol in basic medium will form the thiolate which substitutes the primary bromine. e) Another SN2 the iodide ion is a good leaving group, the final product would be found as an ammonium salt. f) It is an SN1, although the acetate ion is bad nucleophile it is the only ion present.
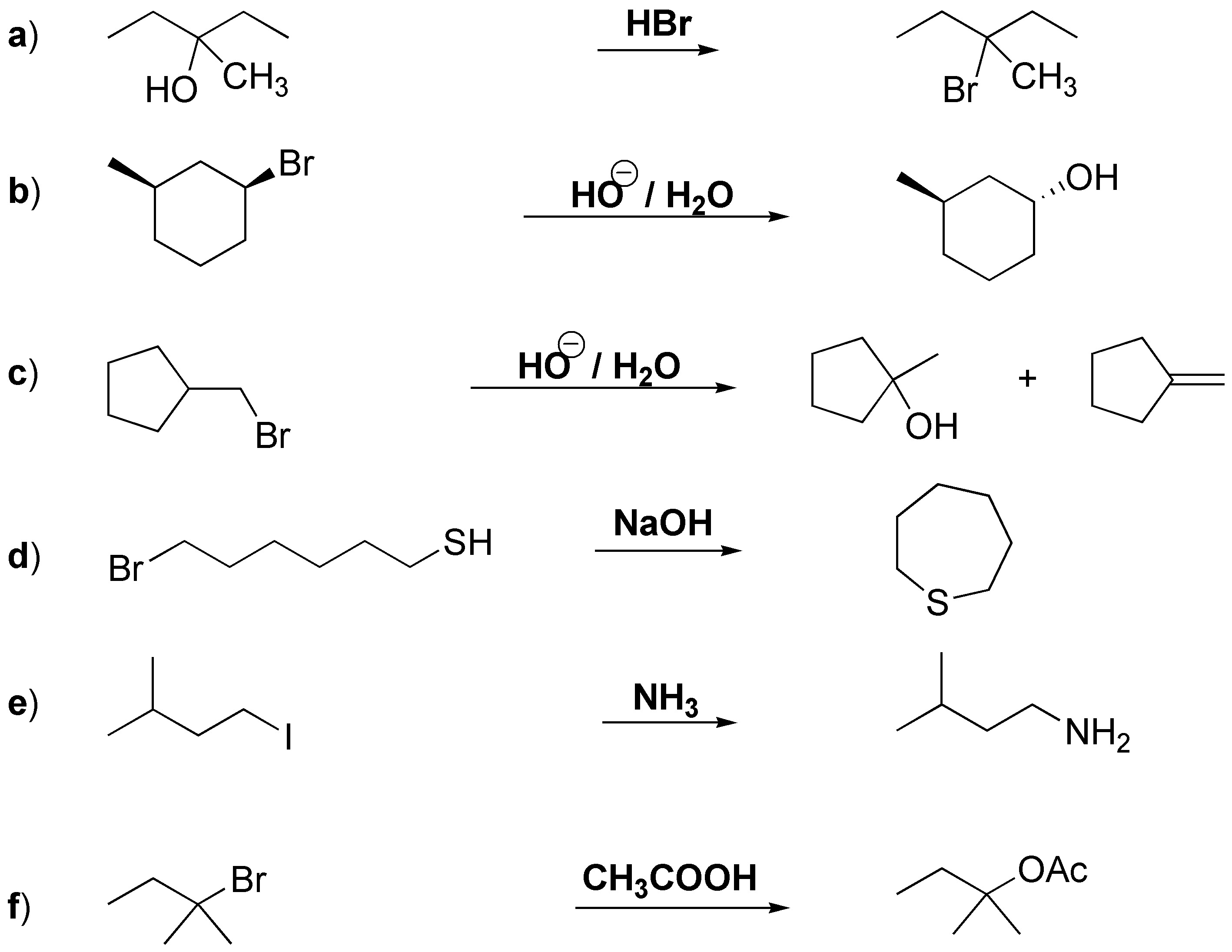
Solution 26:
(a) It is an SN2 as the iodide ion is a good nucleophile and a very weak base; (b) SN2 elimination is not possible; (c) E2, as the methoxide ion is a very strong base; (d) SN1, it is a tertiary haloalkane and water is a very polar and protic solvent; e) SN2 the cyanide ion is a good nucleophile; f) Although the cyanide ion is a good nucleophile the haloalkane is very hindered and no reaction will occur; g) SN2, since it is a primary alcohol, transpositions will possibly occur.
Solution 27:
The starting products are:

In elimination reactions the leaving group and the hydrogen atom being eliminated must be located in an antiperiplanar arrangement, which in the case of cyclohexane rings means that both groups are trans-diaxial.
To appreciate the situation we use a projection method that allows us to know which groups are in axial and equatorial arrangement, so we use the chair representation.
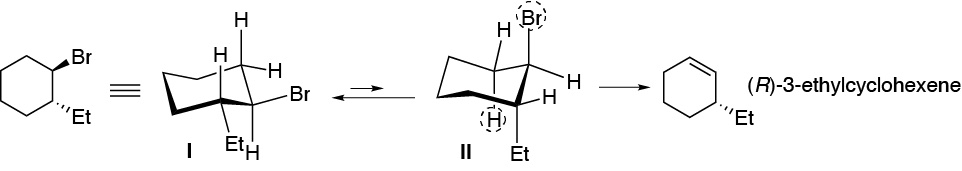
In the most stable confformer (I) the bulky groups, Br and ethyl, are in an equatorial arrangement so it is not possible for the elimination reaction to occur. The compound must evolve to the less stable former II, but with the leaving group in axial and the hydrogen marked with the dashed circle antiperiplanar with respect to it. As a result of the reaction, (R)-3-ethylcyclohexene is obtained. As for (1S,2R)-1-bromo-2-ethylcyclohexane the elimination occurs from the confformer having the bromine atom in axial (III) arrangement. There are two hydrogens which are located antiperiplanar with respect to the leaving group, and which are marked with a dashed circle, and which can give two different alkenes. The major product will be 1-ethylcyclohexene, since it is the most substituted alkene.
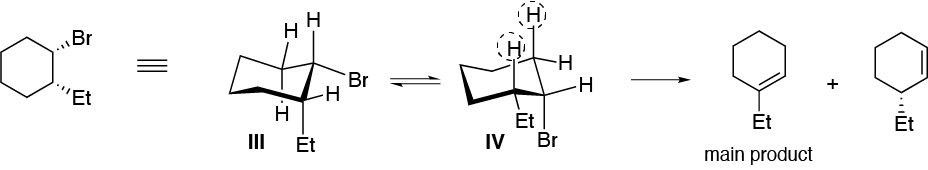
Solution 28:
Only structure II being symmetrical will give a single alkene, the others will give mixtures more or less enriched in the most stable (most substituted, Zaitsev) alkene.
Solution 29:
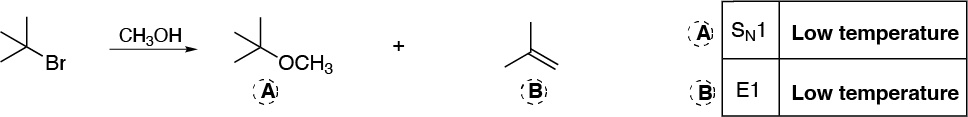
(a and (b): The tertiary structure of the halide suggests a monomolecular mechanism, since the intermediate carbocation is tertiary. The difference between the two reactions lies in the temperature used for each. In this type of substrates, the substitution reaction predominates at low to moderate temperatures, while the proportion of the elimination product is increased at higher temperatures.
c) The substrate is of tertiary type, so bimolecular, SN2 or E2 type mechanisms must be ruled out. The result of the reaction is a mixture of three products:
![]()
The major product must be the solvolysis product, since there is a high proportion of solvent molecules, which can attack the carbocation formed as an intermediate in the reaction.
d) The substrate is secondary, so the reaction mechanism must be bimolecular. The methoxide is basic and its nucleophilicity is moderate. The determining factor in this case is the low temperature, which causes the SN2 substitution reaction to predominate over the E2 elimination.
![]()
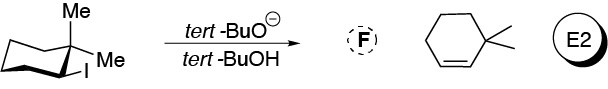
e) A non-nucleophilic base such as potassium tert-butoxide is used as a reagent. The reaction is of type E2, and a single product, cyclopentene, is obtained.
![]()
f) The reaction conditions are typical of a bimolecular elimination process, since a strong non-nucleophilic base is used. The substrate presents two types of hydrogens in position b, with respect to the leaving group, susceptible to be located in the anti suitable arrangement (CH2 and CH3), marked with a dashed circle.
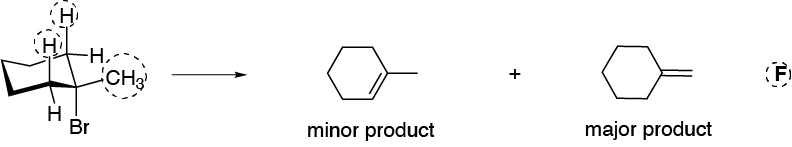
In the first case, a single product would be obtained, since the two axial hydrogens are symmetrically equivalent. The elimination of the bromine atom with a methyl hydrogen leads to the formation of an exocyclic double bond. Potassium tert-butoxide is a bulky base, so steric factors predominate over thermodynamic factors. The reaction product is the least substituted alkene, since the base approaches the primary hydrogens more readily than the secondary hydrogens because they are more accessible.
Solution 30:
(a) The faster reaction is the second reaction. The substrate and nucleophile are the same, but in the second case DMSO, a polar and aprotic solvent facilitates the reaction.
![]()
b) The faster reaction will be the second one since bromine is a better leaving group than chlorine, and all other reaction conditions being equal, it will be the determining factor.
![]()
c) The first reaction is slower than the second. The reaction conditions and the leaving group are the same, but in the substrate there is a branching in position α with respect to the leaving group which makes it more difficult for the nucleophile to approach the bromine-bound carbon.
![]()
d) The faster reaction is the former, since NaSH is a better nucleophile than NaOH, since nucleophilicity increases as one moves down a group in the periodic table.
![]()
Solution 31:
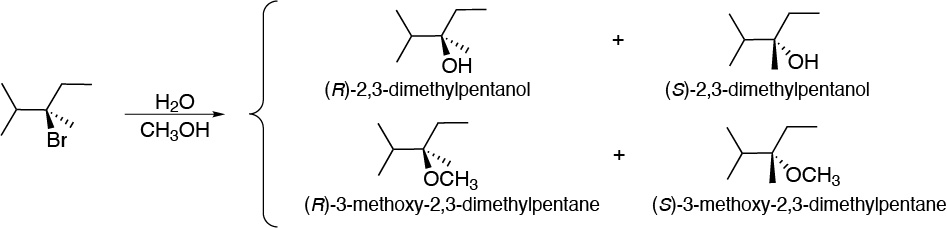
The substrate used in the reaction is a tertiary chiral alkyl bromide and the reagents used have low nucleophilicity. All this suggests a solvolysis reaction. The reaction intermediate is a tertiary carbocation that can be attacked interchangeably by water and methanol so the products obtained will be as follows:
Solution 32:
(a) The reagent needed to obtain the result is acetic acid, since this is a solvolysis reaction (acetolysis) that proceeds by an SN1 mechanism. A racemization takes place if it starts, as in this case, from a chiral substrate.
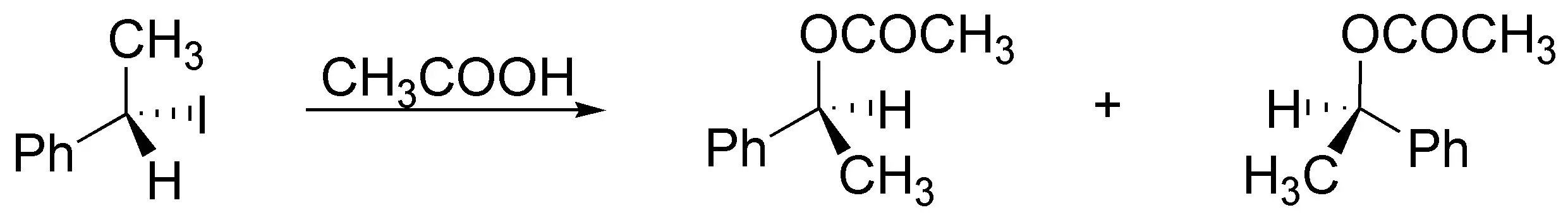
b) The reaction that takes place is an elimination. Since the least substituted alkene is obtained as the reaction product, the necessary reagent is a low nucleophilic and bulky base such as potassium tert-butoxide. The usual solvent in this case is tert-butanol.
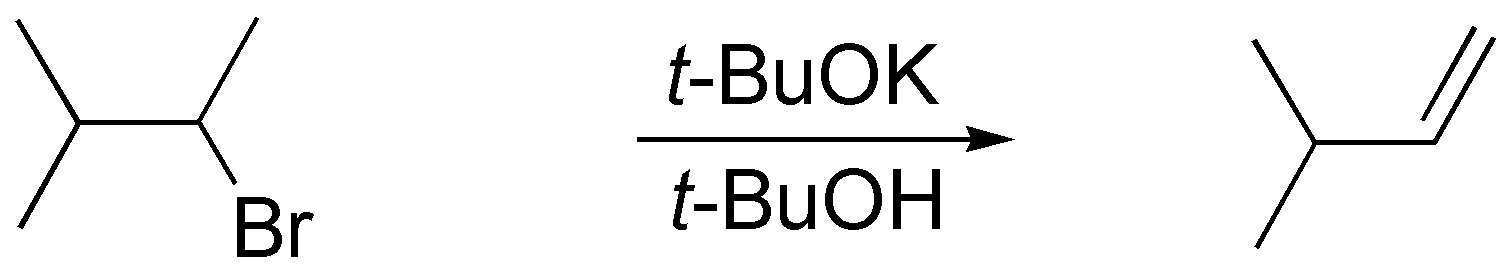
c) Halogen exchange can be carried out by means of a substitution reaction. Since the substrate is primary, the process will be SN2. Sodium or potassium iodide can be used as a reagent and a polar and aprotic solvent such as acetone or DMF is recommended.
![]()
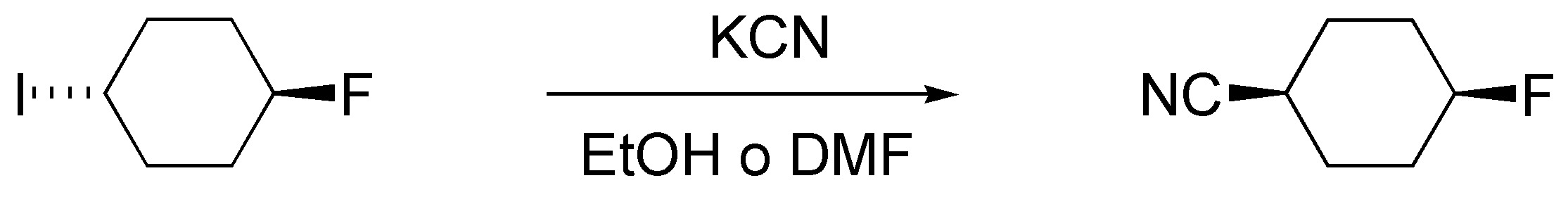
d) Substitution of I by CN takes place, while F, which is a bad leaving group, remains unchanged. The usual conditions for this reaction are the use of the potassium salt of cyanide in a polar solvent such as ethanol or DMF.
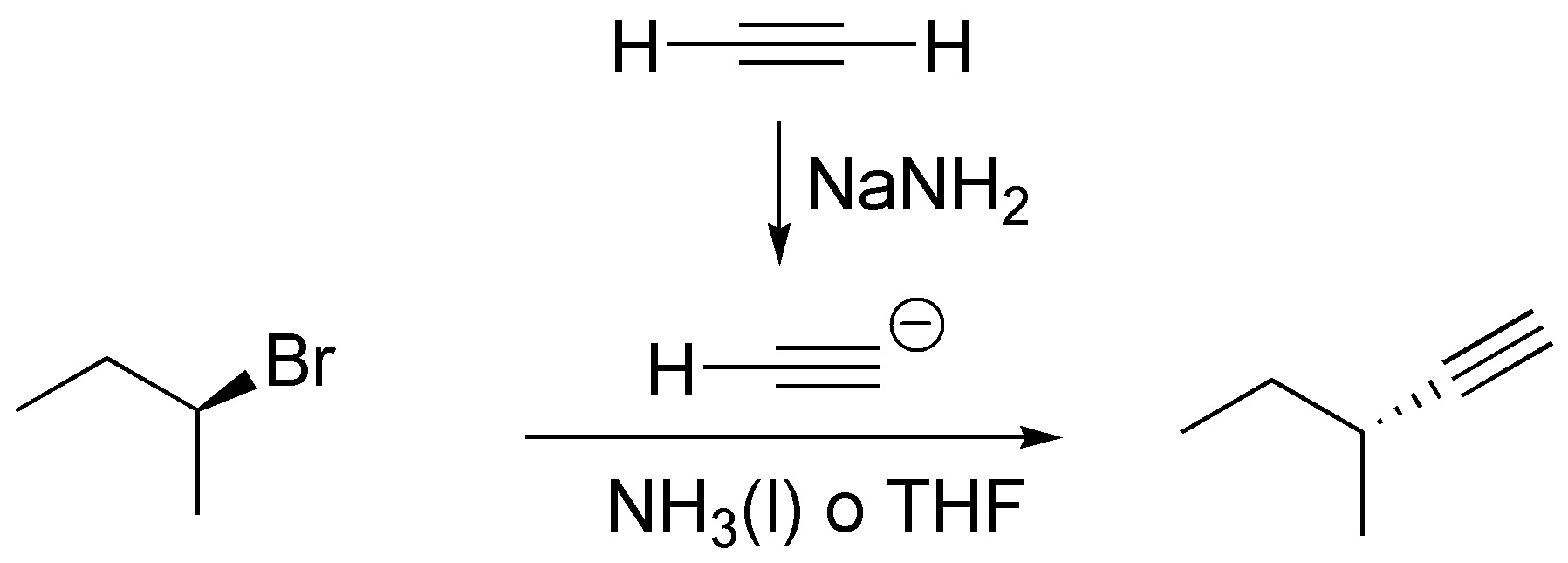
(e) Substitution of Br by the C≡CH grouping is carried out using acetylide, generated from acetylene and NaNH2. Either liquid NH3 which is the conjugate acid of the base (amidide) or THF, a polar and aprotic solvent that prevents the destruction of the base, has to be used as solvent.
f) To produce the least substituted of the possible alkenes requires the use of a bulky base such as potassium tert-butoxide in tert-butanol.
![]()
Solution 33:
The most stable alkene is the most substituted alkene in this case I.
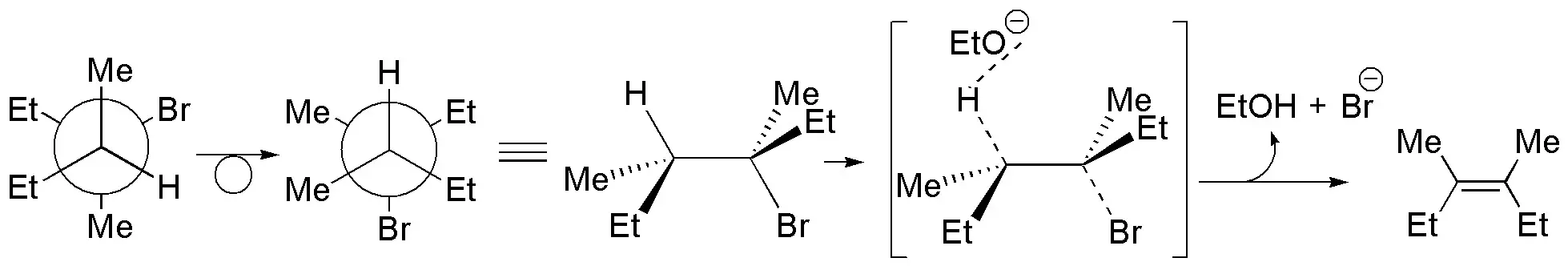
Solution 34:
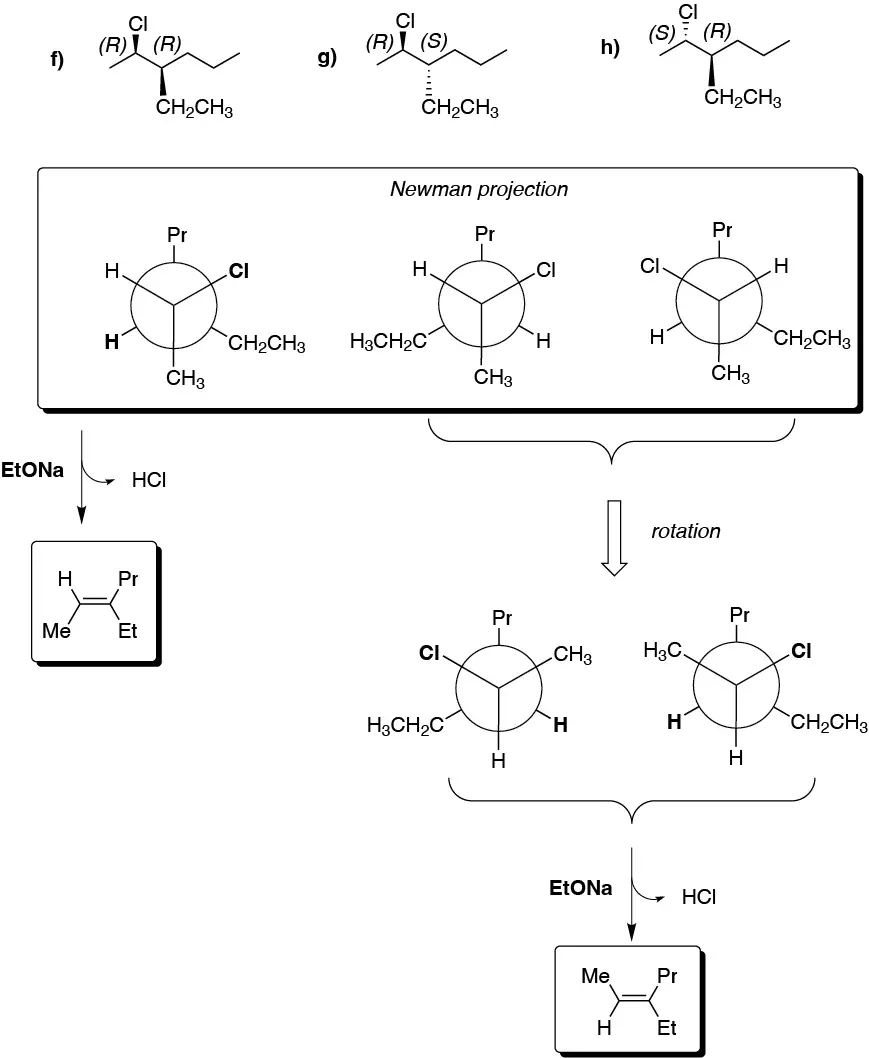
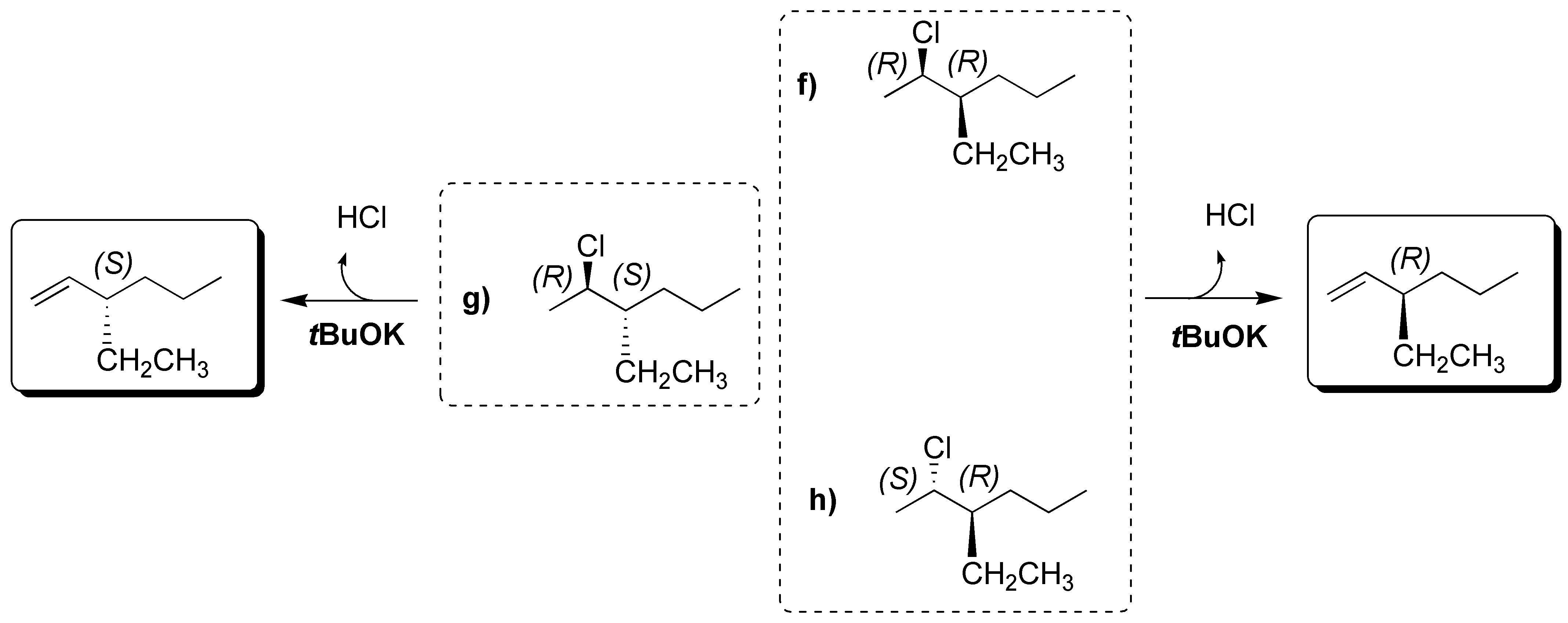
(a) The Newman projection represents (3R,4S)-3-bromo-3,4-dimethylhexane. For the elimination reaction to occur the bromine at C3 and the H at C4 must be antiperiplanar and the appropriate conformation is represented. As a result (Z)-3,4-dimethylhex-3-ene is obtained.
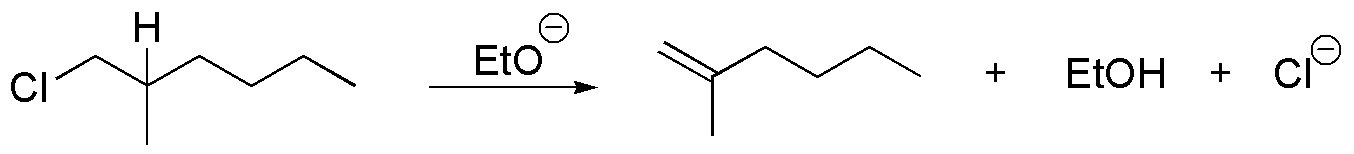
b) There is only one β-hydrogen with respect to the leaving group, which is located on a primary carbon. The alkene formed is:
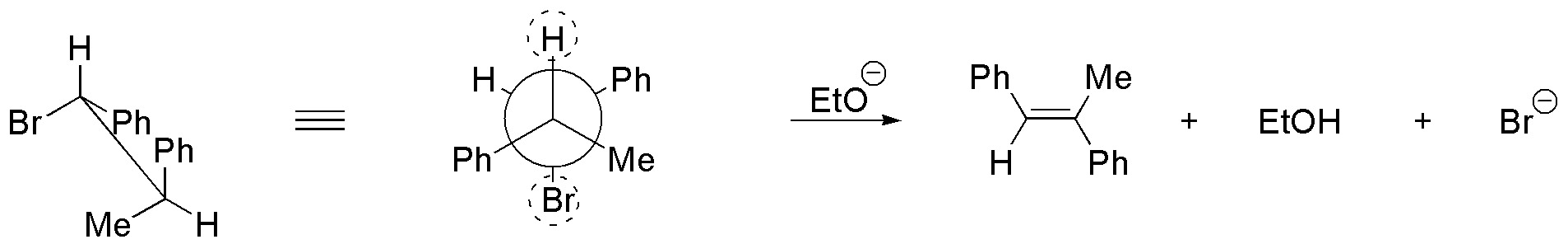
(c) There is no E2 reaction since it does not have hydrogens in proper arrangement.
d) The conformation represented does not have the groups to be eliminated in the proper arrangement, so the molecule must be rotated around the single bond until the bromine and the hydrogen of the adjoining carbon are antiperiplanar.
e) The compound has two hydrogens that can be antiperiplanar with respect to the leaving group. We proceed to draw the conformers in which this situation occurs, from which the corresponding alkenes are obtained. II is more stable than I, since the Et- and Me-groups, which are the bulkiest are placed as far apart as possible, so that the major product will be (E)-pent-2-ene.
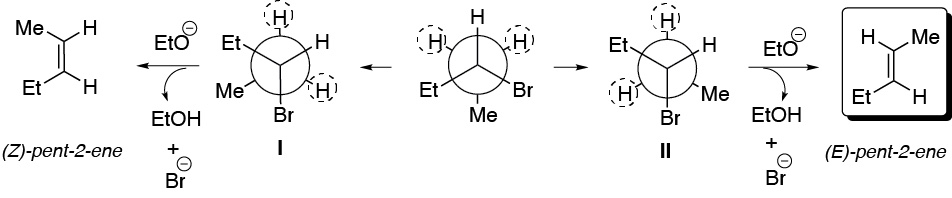
Solution 35:
The most stable conformers of I and II are those in which the isopropyl group, which is the bulkiest presents an equatorial arrangement. Moreover, for BrH to be removed and the double bond to form, Br and H must be in an antiperiplanar position. For the cis-isomer the bromine is antiperiplanar with respect to two hydrogens, so that from this conformation the elimination reaction can take place, whereas for the trans-isomer a conformational change is required, precisely towards a particularly disfavored confomer. Hence the difference in the reaction rate.
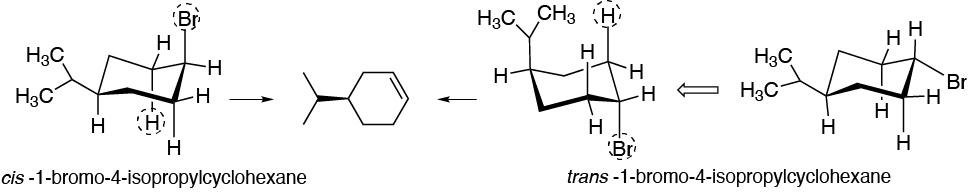
Solution 36:
The reaction scheme is as follows:
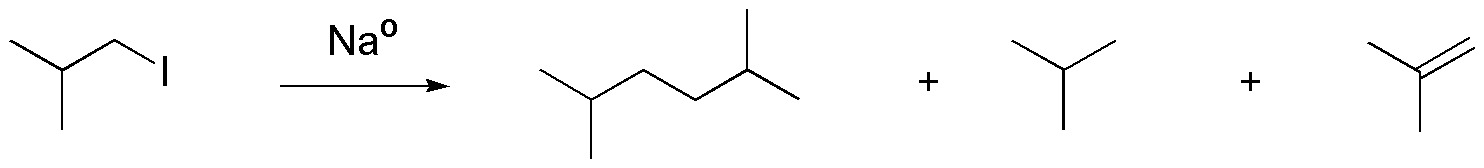
and corresponds to an example of the Wurtz synthesis.
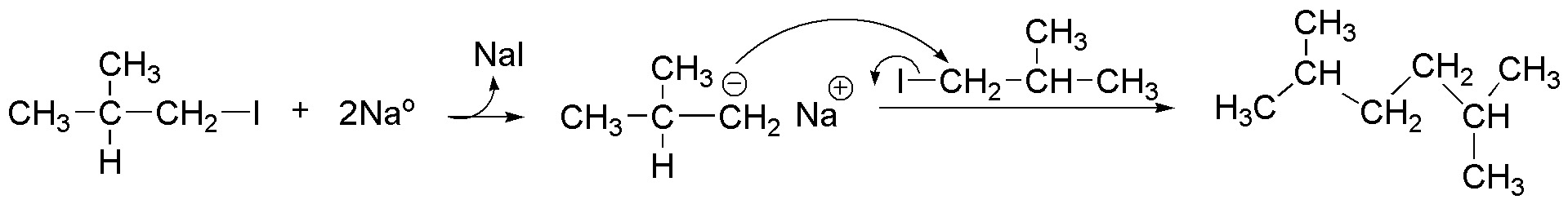
The formation of the by-products can be justified by considering the basic character of the intermediate anion, capable of abstracting a proton from the starting alkyl iodide, transforming it into an alkane. Simultaneously to the loss of the proton, the departure of the iodide occurs, thus forming a double bond.
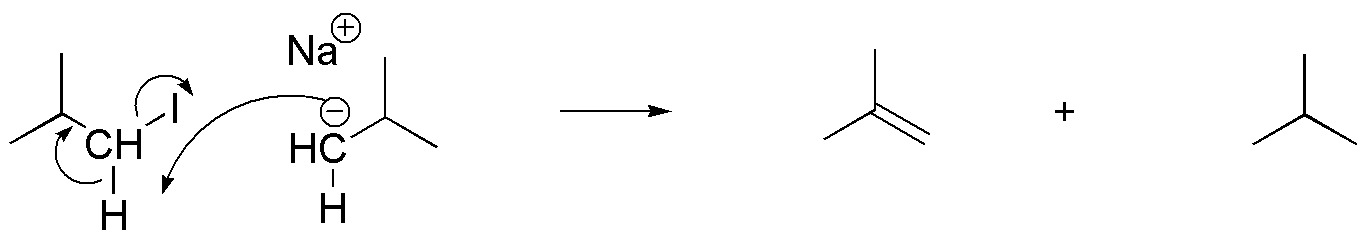
Solution 37:
(a) The result of the reaction will be the formation of cyclopentane. The dehalogenation is carried out radically with (n-Bu)3SnH and requires the presence of an initiator (AIBN) to start the process.
![]()
b) The sequence of reactions described corresponds to an example of Corey-House synthesis. In the first step of the reaction an organolytic is produced, which treated with copper generates a lithium dialkylcuprate, B1, (Gilman’s reagent). In this first step the starting halide can be primary, secondary or tertiary. Subsequently, this lithium dialkylcuprate can be treated with a primary or secondary alkyl halide, which can be cyclic or acyclic. In this case it corresponds to cyclopentyl bromide. As a result, an alkane is produced from the bond between the fragments used.
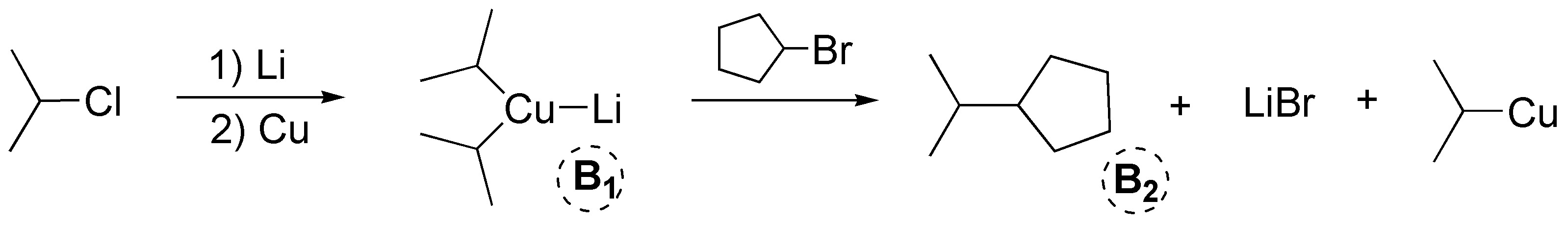
c) and d) Both are intramolecular Wurtz reactions. In this case the formation of by-products is minimized when two different alkyl halides are used. The result of both reactions is as follows:

Solution 38:
a) and c) are two SN1, in the first one the iodide ion is an excellent nucleophile (as well as good leaving group) and in the third one the solvent acts as such. b) is a SN2, the substituted bromine is the primary, the vinyl is very unreactive. d) is another SN2with inversion of the configuration.
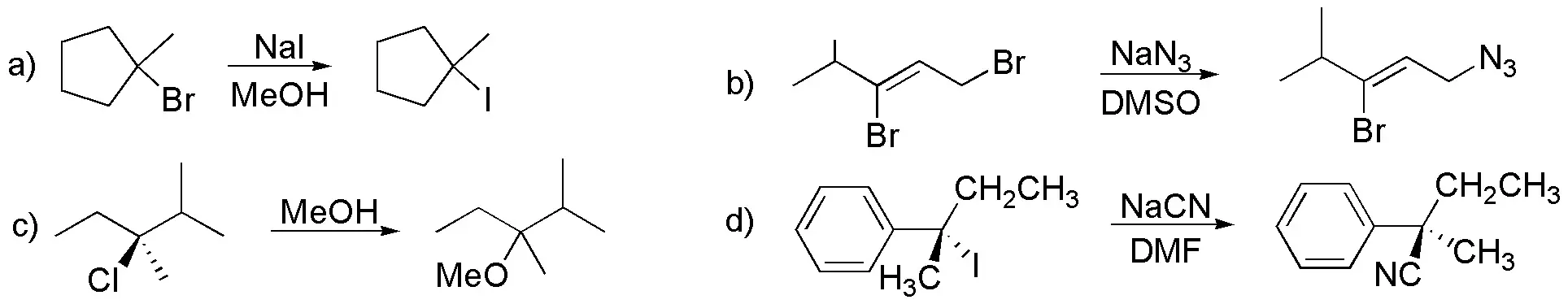
Solution 39:
In SN2 the primary halides react better than the secondary ones and these much better than the tertiary ones. As for the primaries a) and c) the one with the best leaving group reacts better: iodide better than bromide and chloride. So the order will be:
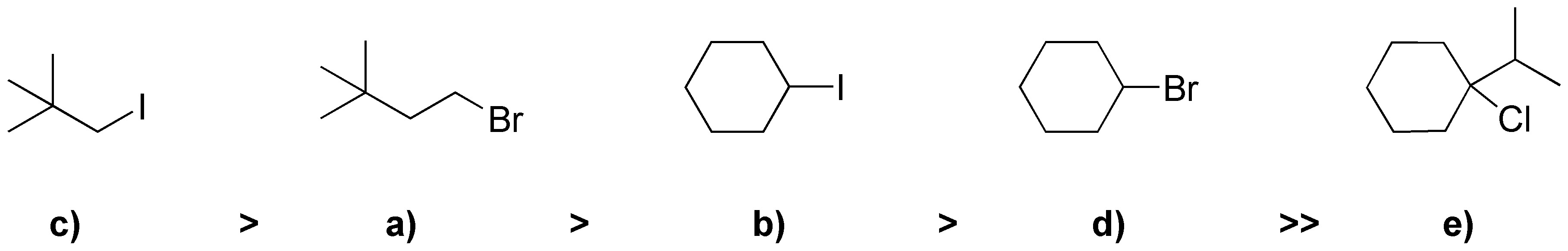
Solution 40:
(a) NaBr / MeOH, since this is a racemization of a tertiary haloalkane.

b) EtONa / EtOH (low temperature) because it is a SN2.
![]()
c) NH3; it is a double SN2.
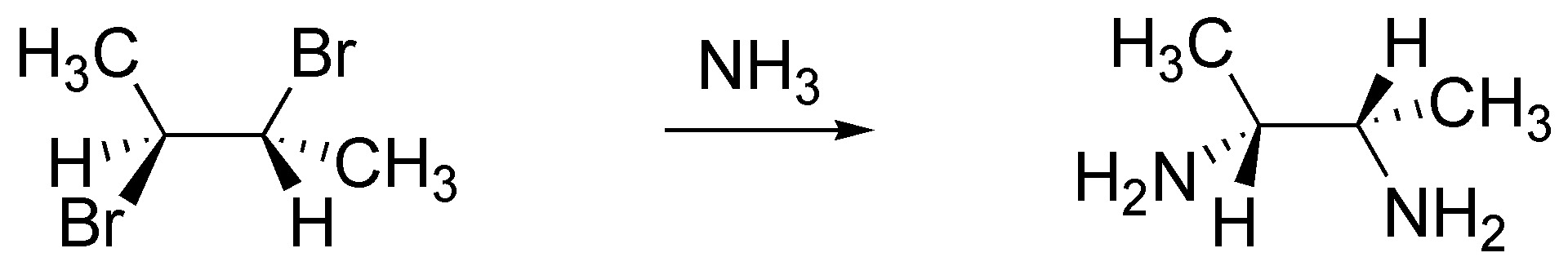
d) NaOH. It is a double SN2 the first intermolecular and the second intramolecular in which the nucleophile is the alkoxide or alcoholate generated by the soda.
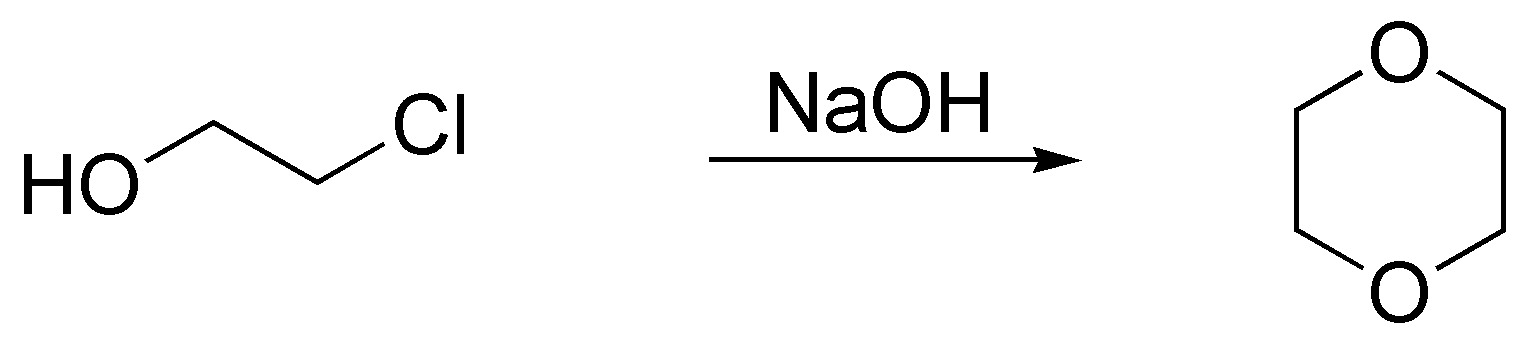
Solution 41:
The rate of solvolysis is higher for tertiary than for secondary haloalkanes and is also favored by the nature of the leaving group (iodide better than bromide). So the order of solvolysis rate will be:
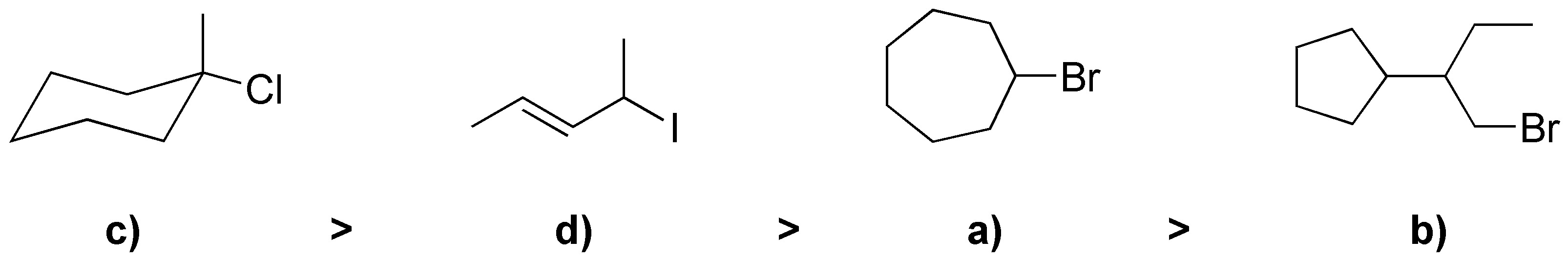
Solution 42:
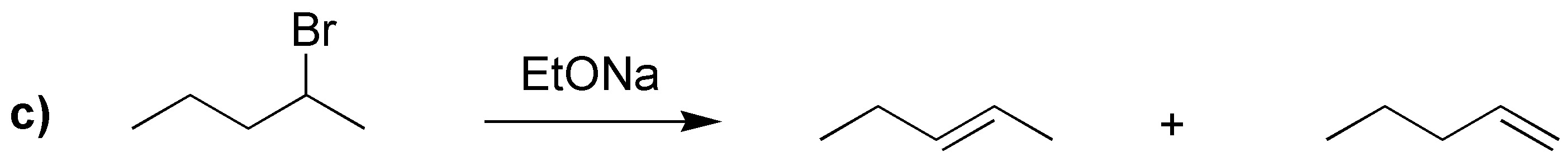
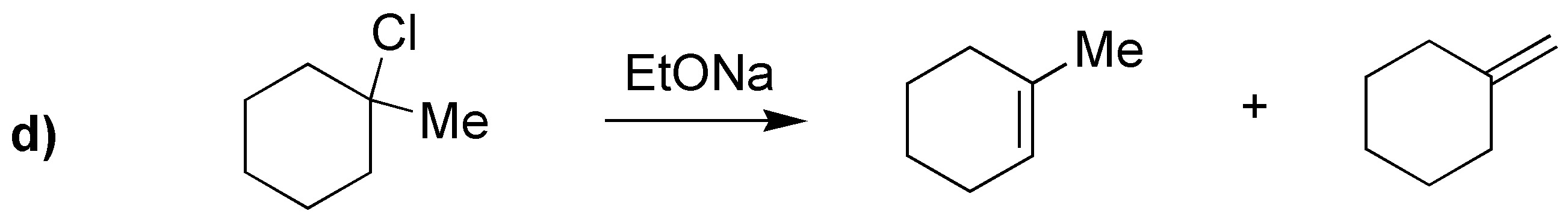
a) E2 will be produced since it is treated with a strong base, SN1 will not be produced. b) By not using a base SN1 will be produced. c)Of the two halogens bromine is much better leaving group; since the nucleophile is also a strong base a mixture of E2 and SN2 will be produced. d)Using a strong base and also very bulky will produce an E2 giving the least substituted alkene. e)E2 elimination will produce a mixture of alkenes. f)An E2 will be produced even if a bulky base is used.
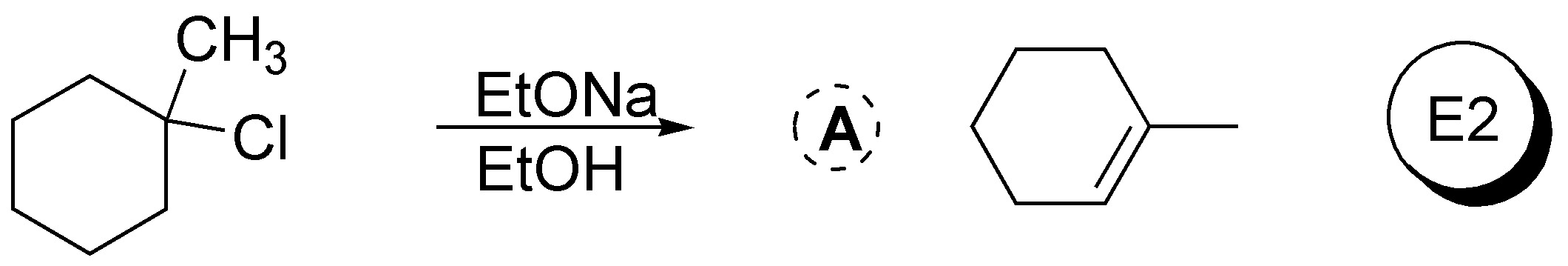
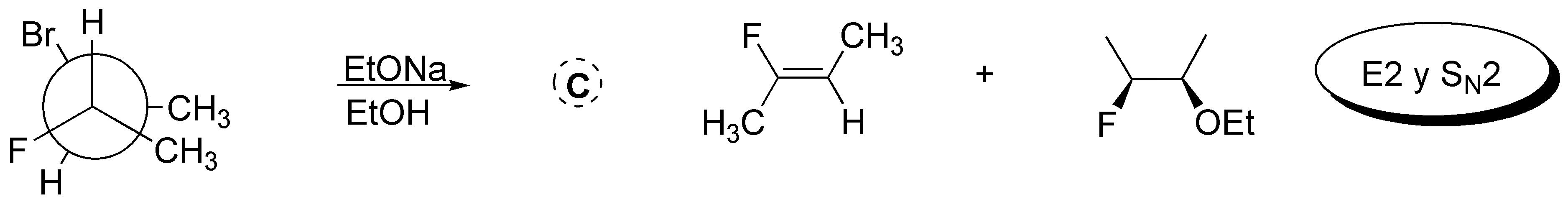


Solution 43:
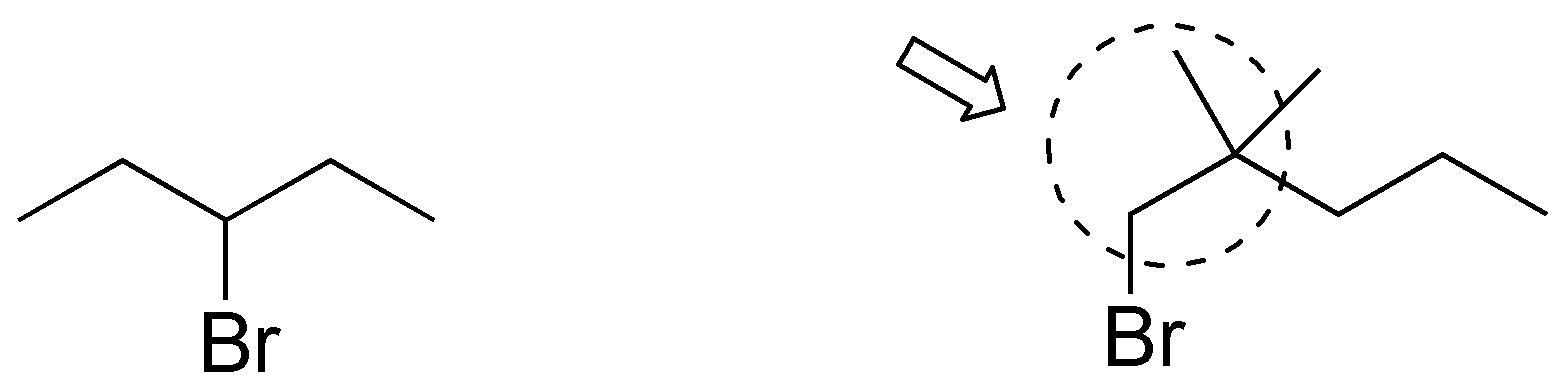
3-bromopentane exhibits a higher nucleophilic substitution reaction rate than 1-bromo-2,2-dimethylpentane as the methyl groups in position 2 of the latter hinder the approach of nucleophiles in SN2 type reactions.
Solution 44:
In the case of the cis isomer the most substituted alkene is produced, as predicted by Zaitsev’s rule, but in the trans isomer because it is necessary that the groups to be removed (Br and H) have an antiperiplanar arrangement and in this isomer it only has suitable hydrogen at position 6 of the ring.
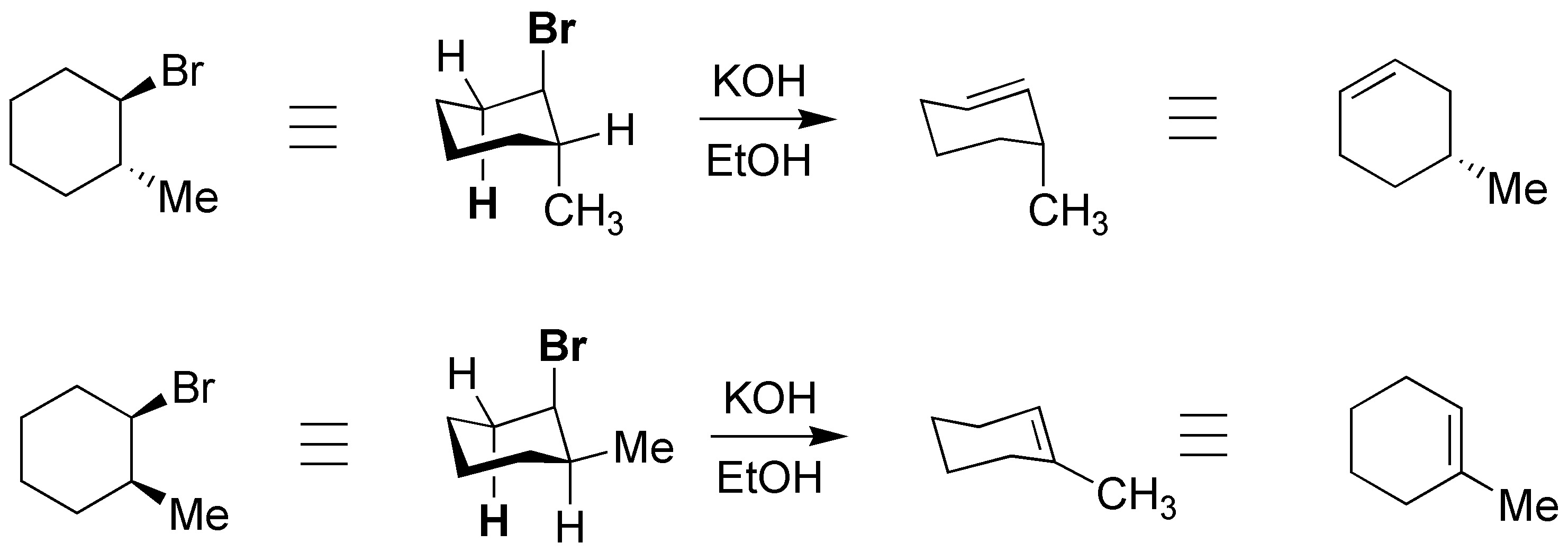
Solution 45:
The cause of the higher velocity of one isomer over the other is the different stability of the conformations that the products have to adopt for elimination to occur. Bromine and hydrogen in antiperiplanar arrangement, as shown in the figure:
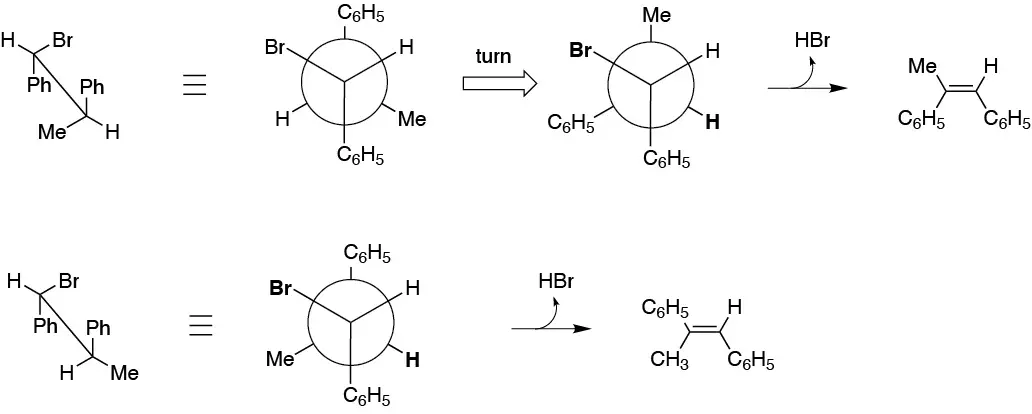
Solution 46:
The difference between the use of one base or the other is going to be translated mainly in the nature of the alkene mostly produced: the tert-butoxide being more bulky usually produces the attack on the less impeded carbon giving mostly the less substituted alkene. Not so in the case of the ethoxide in which the most substituted (thermodynamically more stable) predominates and can even give substitution.
In case a) elimination cannot occur, then the corresponding nucleophilic substitutions will occur:

With sodium tert-butoxide the corresponding alkenes with the terminal double bond are obtained.
Solution 47:
For bimolecular elimination to occur the chlorine and hydrogen must be in an antiperiplanar arrangement. As can be seen in the scheme in the first case there is only one hydrogen in the proper arrangement so that only one product is produced. Not so in the second, in which there are two:
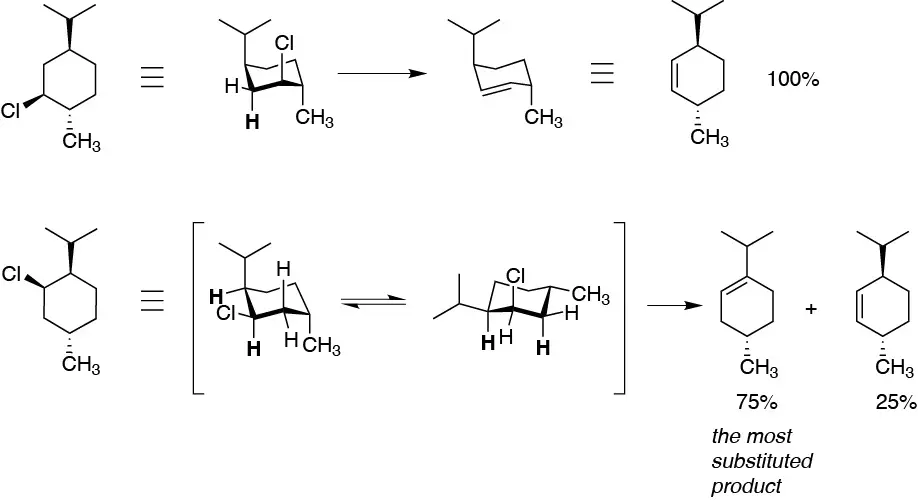
Solution 48:
The iodide ion is a good leaving group and although acetic acid is a bad nucleophile it can produce the substitution reaction:
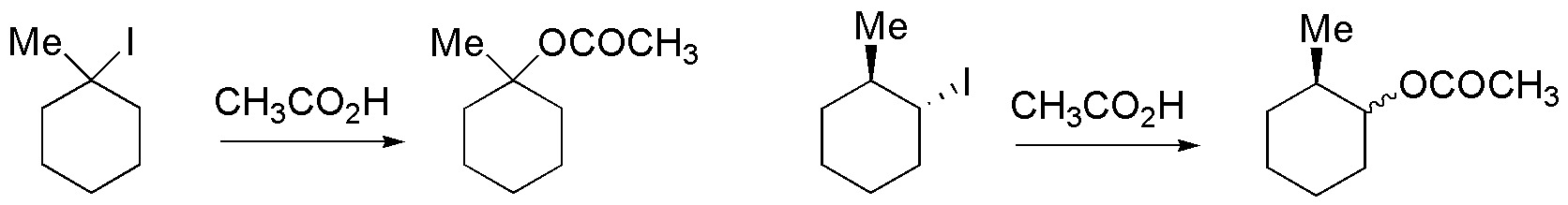
Solution 49:
a) Treatment with alcoholic potash will produce an E2, which is a stereoselective reaction, so both compounds will give different diastereoisomers of the most substituted alkene (E and Z). b) With potassium tert-butoxide, an elimination will also occur giving in this case the least substituted alkene. The two enantiomers of this alkene will be produced, maintaining the configuration of the chiral carbon that is not involved in the elimination. c) With sodium aside, SN2 will be produced. As it is a stereospecific reaction each of the stereoisomers will produce a different diastereoisomer. d) With tri-n-butyltin hydride dehalogenation occurs giving the corresponding alkane, each of the diastereomers will produce a different enantiomer.
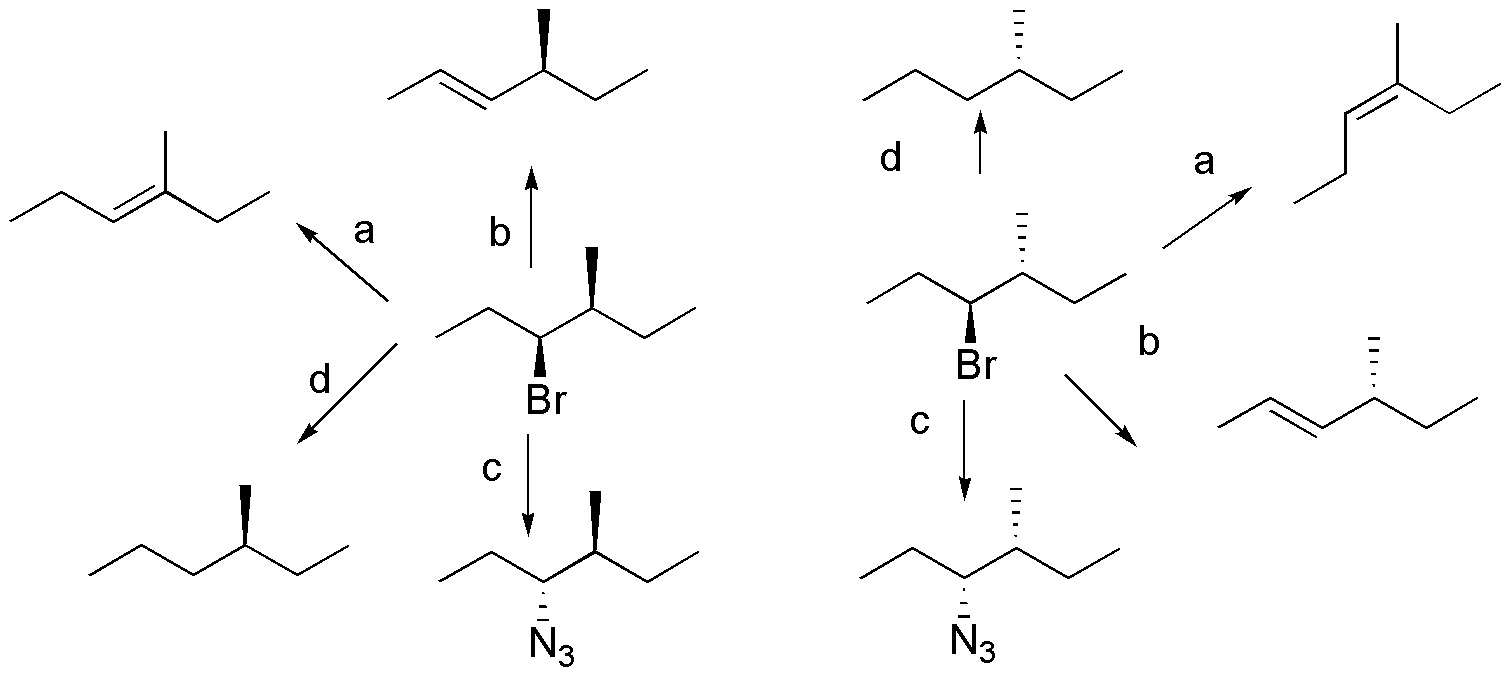
Solution 50:
According to Zaitsev’s rule the more substituted alkene will be more stable therefore.
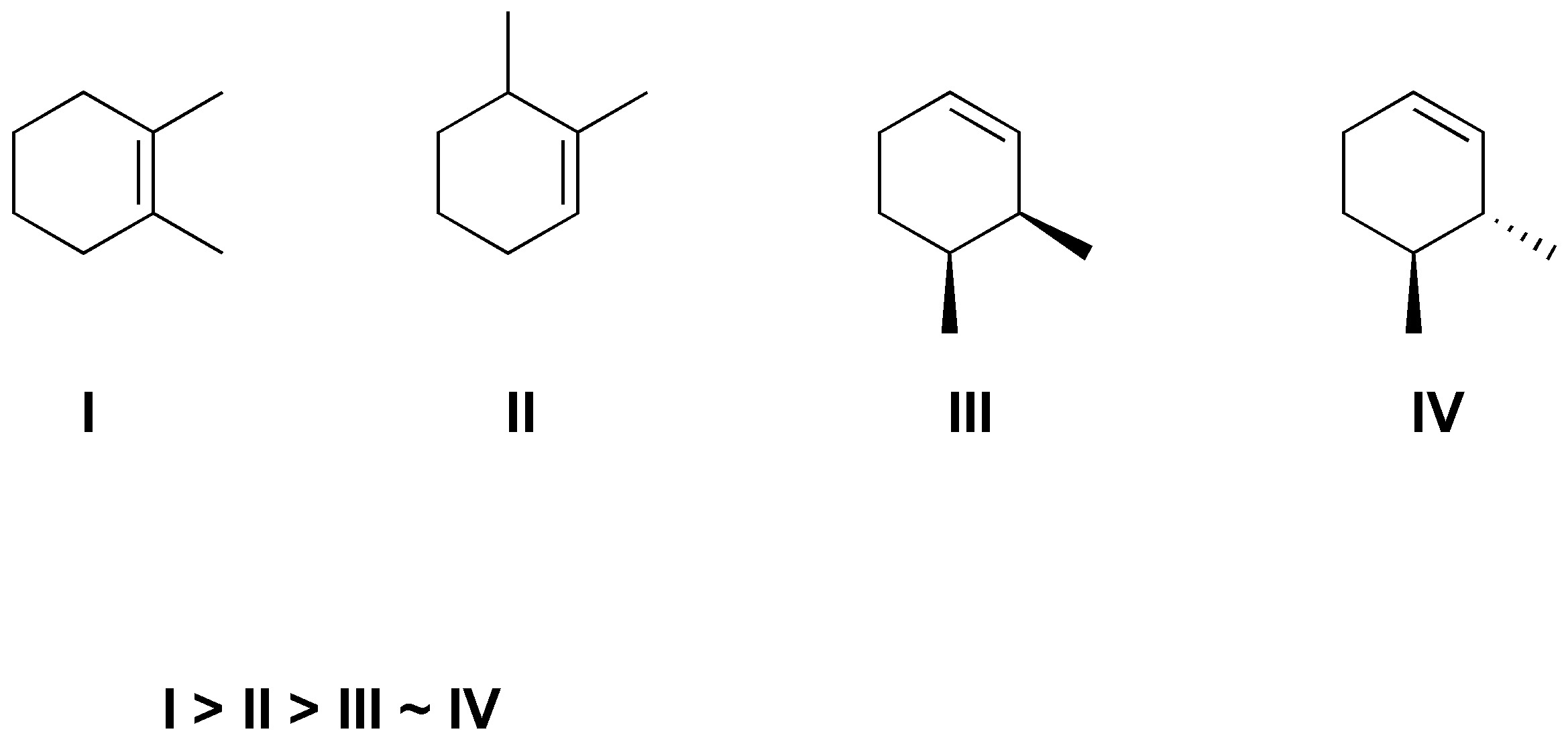
Solution 51:
The options chosen for the eliminations are: a) The less substituted alkene will be produced. c) As it is required that the bromine and the hydrogen to be eliminated are in antiperiplanar arrangement the only solution is the one indicated. d) By the same requirement above the Z alkene will be produced. e) We will have two hydrogens in proper arrangement with respect to iodine, the more substituted alkene will be produced as it is more stable. f) By treating with t-BuOK the less substituted alkene will be produced in this case the exocyclic one.
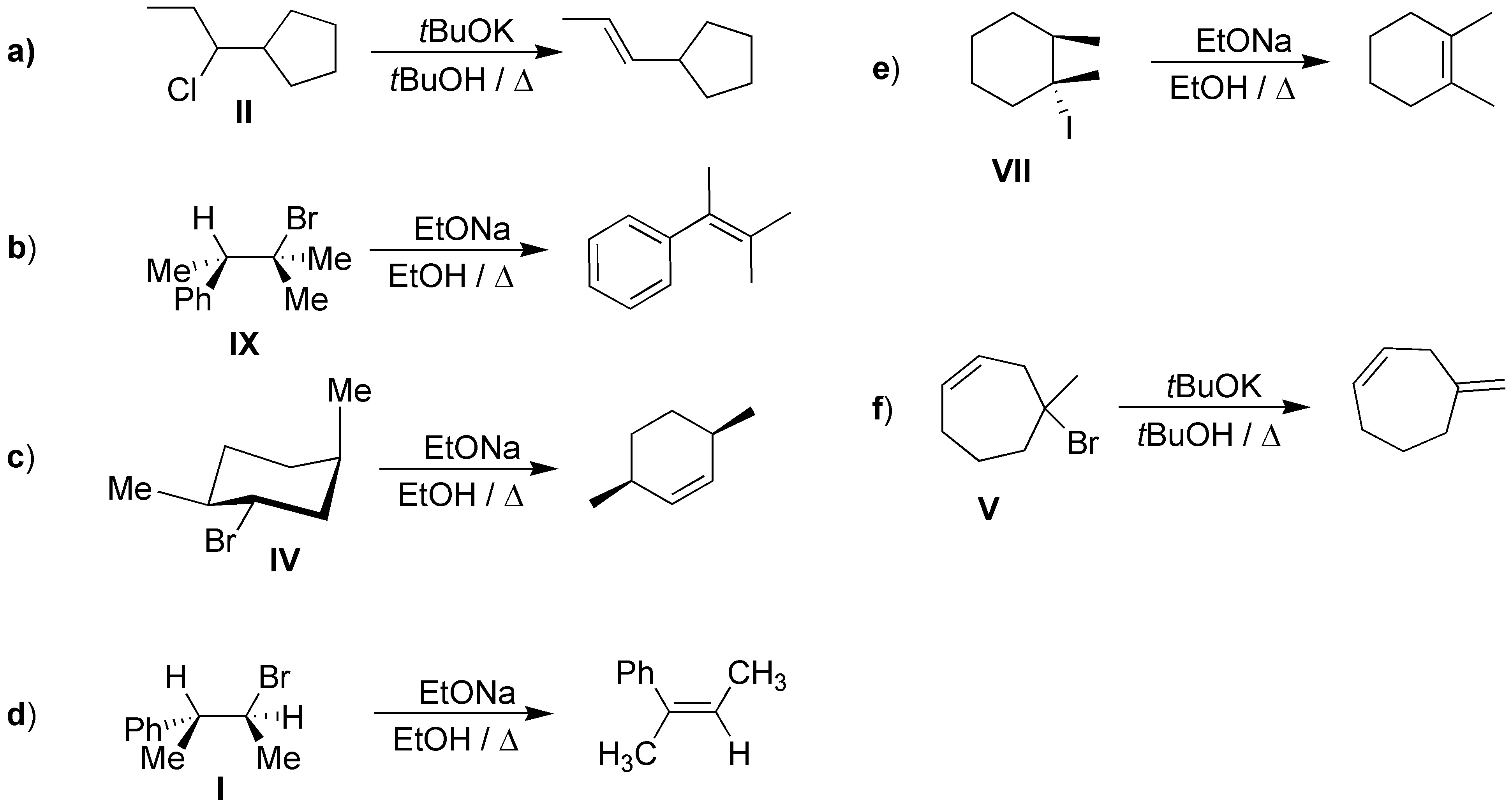
Solution 52:
For bimolecular elimination to occur the chlorine and hydrogen must be in an antiperiplanar arrangement. As can be seen in the scheme, the arrangement of hydrogens is anti with respect to chlorine, leading to two distinct products.
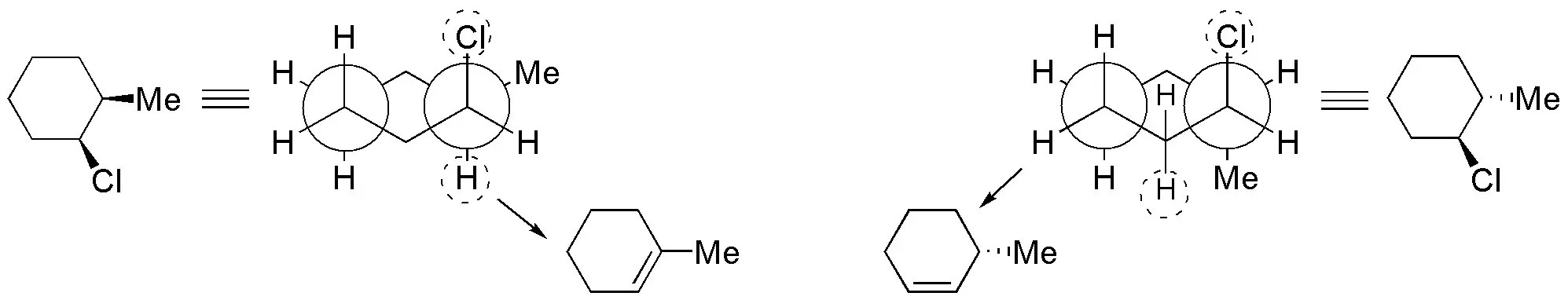
Solution 53:
The only restriction of the Corey-House synthesis is the non-use of a tertiary haloalkane in the last stage of the synthesis, so some possible strategies could be:
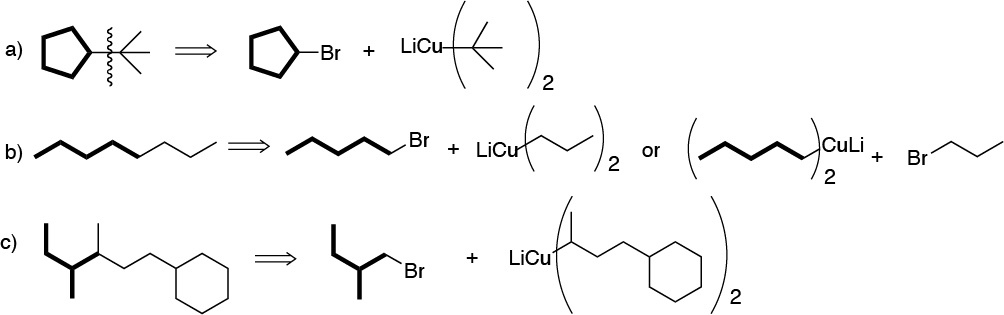
Solution 54:
Two Corey-House syntheses are involved:
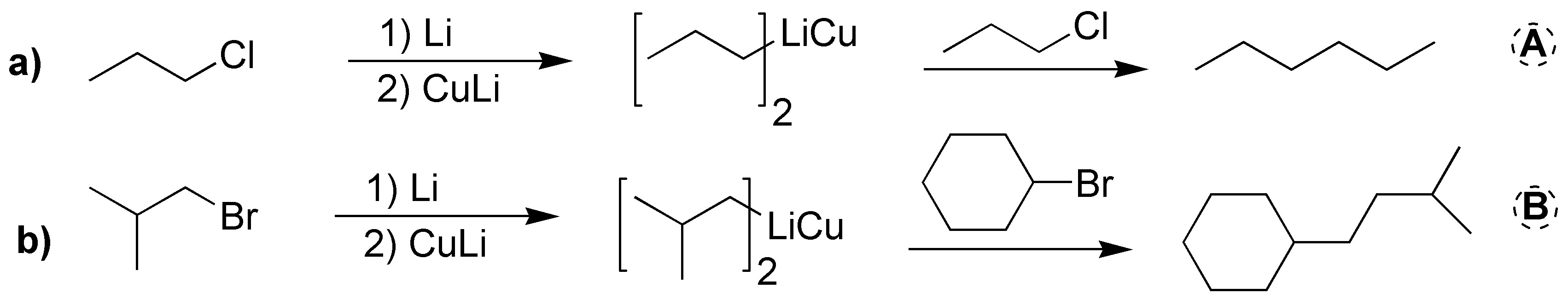
Solution 55:
The only restriction of the Corey-House synthesis is the non-use of a tertiary haloalkane in the last stage of the synthesis, so some possible strategies could be: