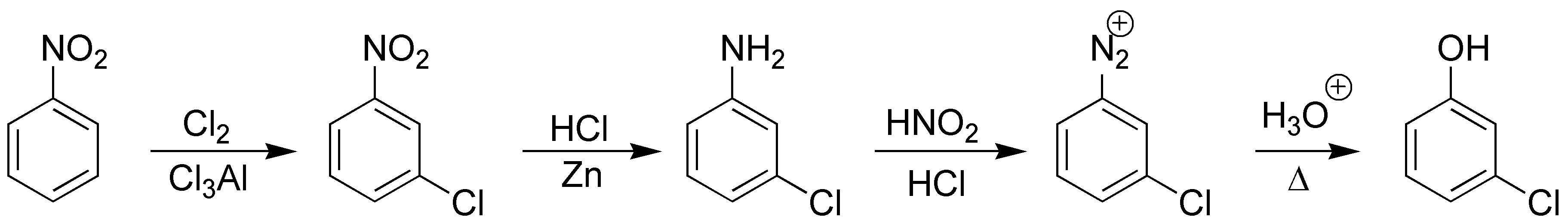
Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Transformations in Substituted Aromatic Compounds – solutions to problems
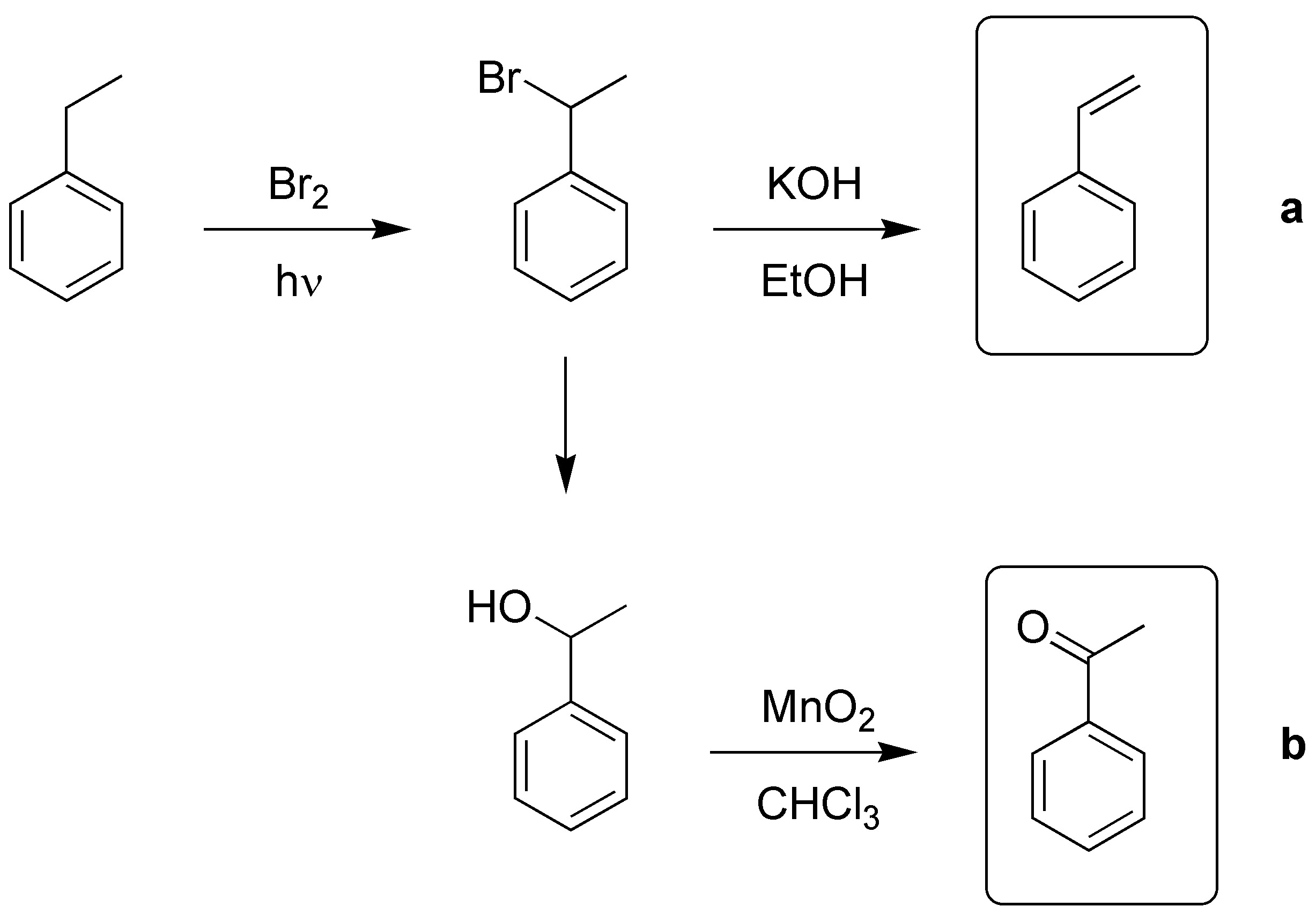
Solution 1:
The key to both transformations will be the functionalization of the benzyl position and the best way to achieve this is by radical halogenation, elimination with alcoholic potash will produce styrene a) and nucleophilic substitution with water will give us the benzyl alcohol which by subsequent mild oxidation with manganese dioxide will give us the carbonyl compound (acetophenone) b).
Solution 2:
Oxidation of methyl to carboxylic acid will occur:
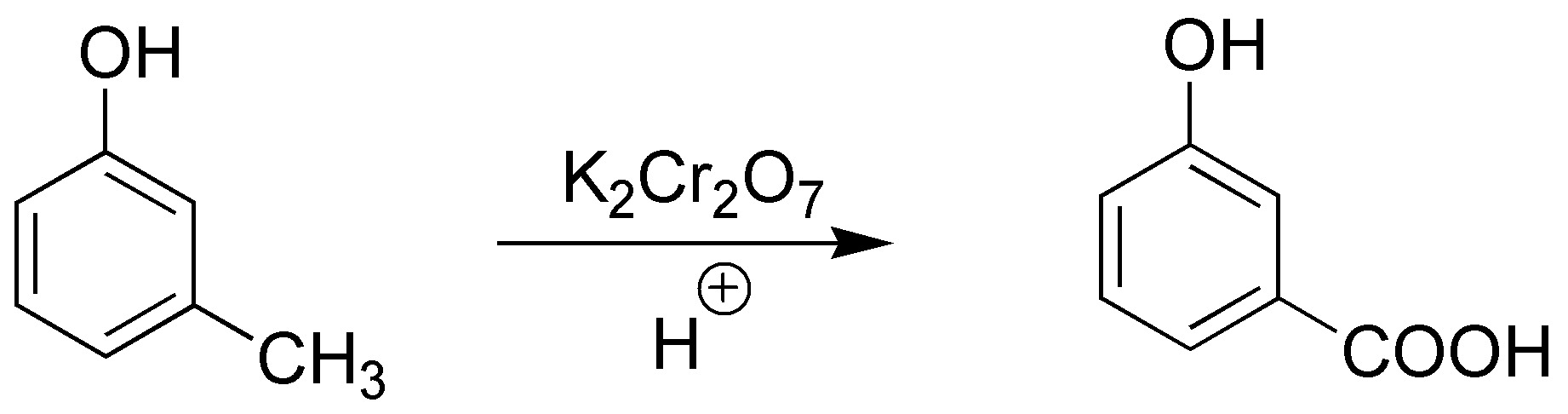
The -OH group is not altered in this reaction.
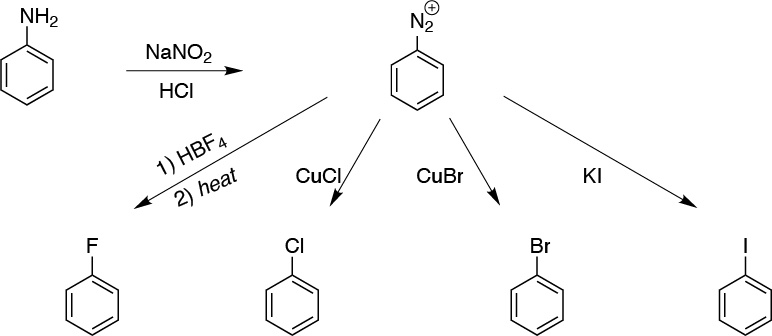
Solution 3:
The best way is via the diazonium salt, these in the presence of cuprous salts of the corresponding halides for Cl and Br allow the halobenzenes to be obtained (Sandmmeyer and Rosenmund type reactions). I– is introduced via KI and F– requires the use of tetrafluoroborate.
Solution 4:
Diazonium salts are excellent electrophiles so they will react with other aromatic compounds giving diazocompounds:
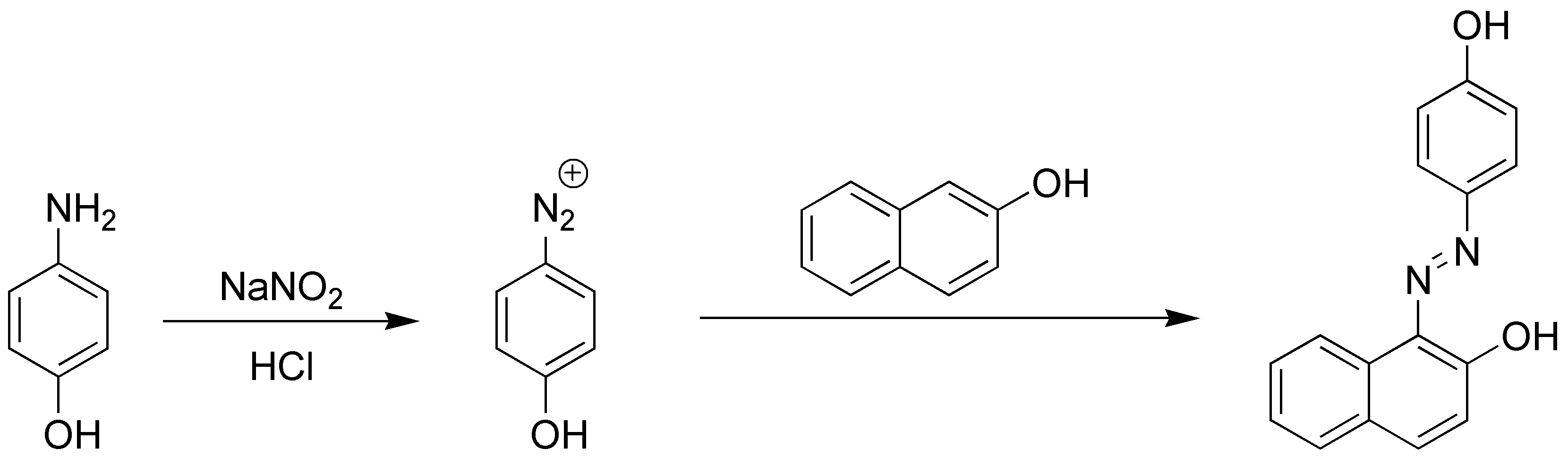
The electrophile attack preferentially occurs at position 1 of the naphthalene.
Solution 5:
Another interesting application of diazonium salts is the conversion to sulfides by reaction with thiols:
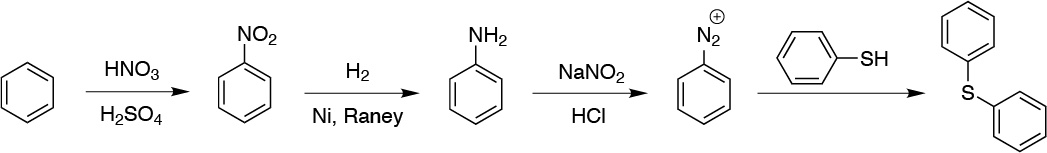
Solution 6:
a) From benzene bromobenzene can be obtained, which by Friedel-Crafts alkylation gives us the desired product together with the ortho-isomer.
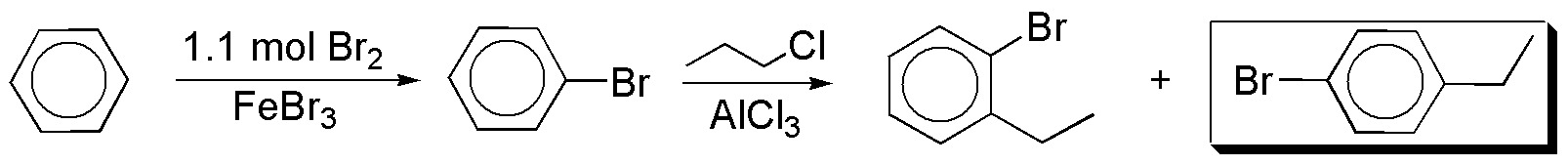
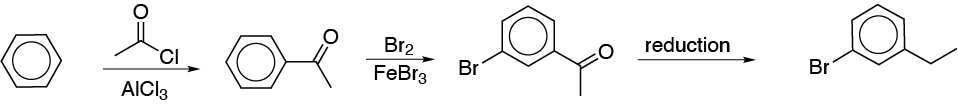
b) For the introduction of the groups in a relative meta– arrangement a different synthesis strategy is required. When an alkyl chain and a meta-group are available, the usual procedure is to perform a Friedel-Crafts acylation reaction, followed by a second electrophilic aromatic substitution, to introduce the desired group. The carbonyl group is then reduced with a reagent compatible with the functions present in the aromatic compound.
c) The key to the preparation of the desired compound lies in:
- A carboxyl group can be generated by oxidation of an alkyl chain.
- Introducing a group in the ortho-position before transforming that chain into a carboxyl since COOH is meta-director.
- Blocking the para-position avoids obtaining the corresponding isomer. The conventional way to block a position in a benzene ring is by a sulfonation reaction, since the SO3H group can be removed by gentle heating in an acidic medium.
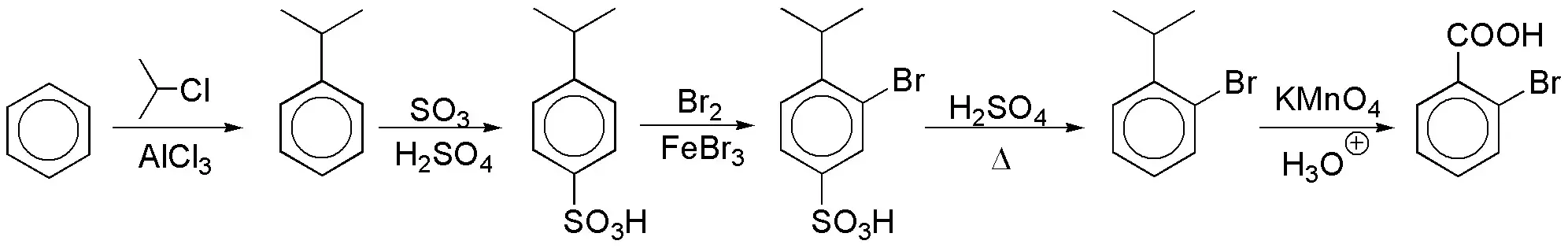
In the first reaction a bulky group has been used in order to favor the para-orientation in an exclusive way, avoiding by steric effects the formation, in this step, of the ortho-isomer.
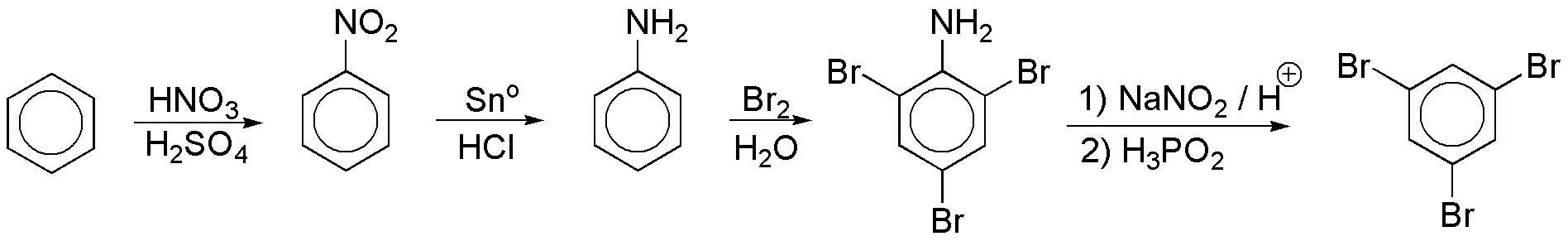
d) Polysubstitution reactions are favored with strongly activating and ortho– and para-directing groups. In this respect, OH and NH2 groups could be useful. These should be eliminated in a second step of the synthesis. NH2 has these characteristics, since diazonium salts are hydrolyzed with H3PO2, and these salts are obtained from the NH2 groups.
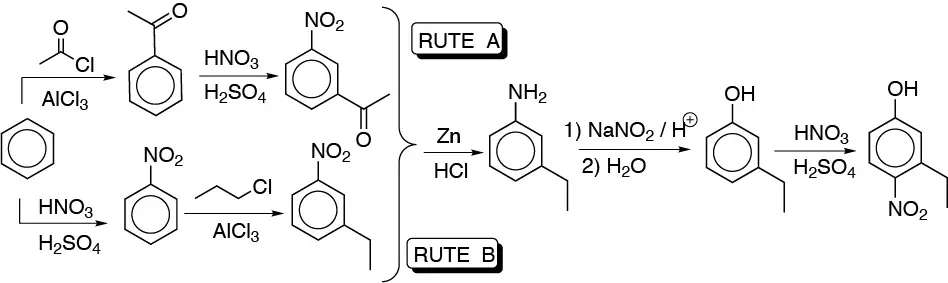
e) Two routes can be followed for the synthesis of this compound:
The first route is more favorable, since the COCH3 group is moderately deactivating, while the NO2 group is strongly deactivating.
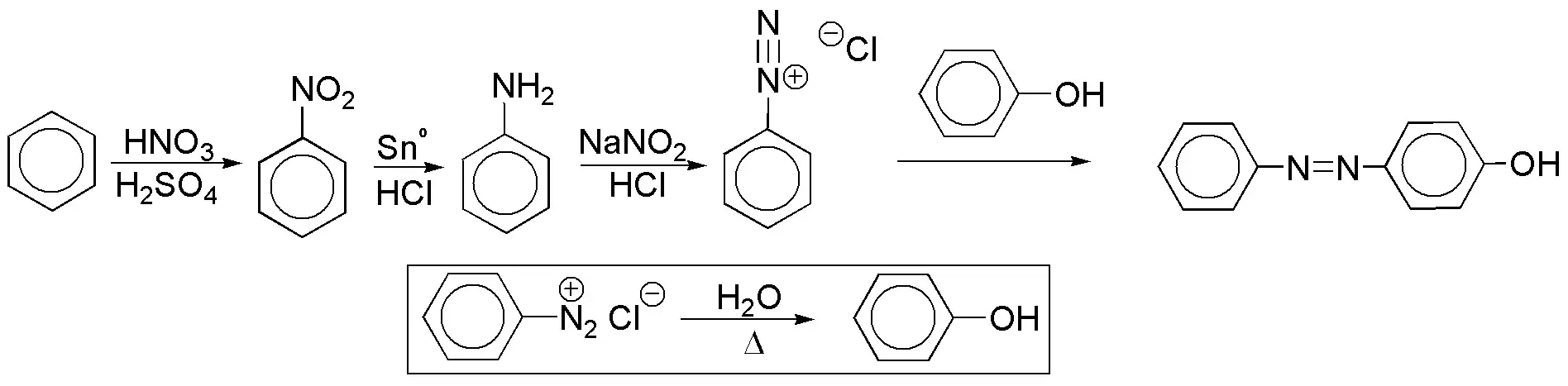
f) The azocompounds are prepared with the aid of diazonium salts, which in turn are obtained from amines. Benzene can be used as the only starting product, since phenol, which is necessary for obtaining the diazocompound, can be prepared from the diazonium salt by gentle heating in aqueous medium.
Solution 7:
The first reaction is a nitration, so that A is nitrobenzene. Then the nitro group is reduced with Zn and HCl. The result is aniline chlorohydrate, since the medium is acidic. To isolate aniline B it is necessary to treat with a base. Then the amine is acylated with acetic anhydride and a base or with acetyl chloride, also in the presence of a C base. In this way, the reactivity of the aniline is dampened and the amino group is protected against the next step, which is a new nitration reaction with nitric acid catalyzed with sulfuric acid D. Once the NO2 group has been introduced, the NH2 is released from its protection by means of acid or basic hydrolysis. Acid hydrolysis has the disadvantage that it is necessary to treat with a base to release the amine, since what is really isolated in this step is the chlorohydrate of the amine, so basic hydrolysis, E (HO–, H2O), is chosen. With sodium nitrite in an acid medium the diazonium salt F is produced. The reaction is carried out at low temperature, since this type of compounds are unstable and decompose easily. Finally a CN group is introduced, by means of a Sandmmeyer reaction, for which the corresponding copper(I) salt of cyanide G is used.
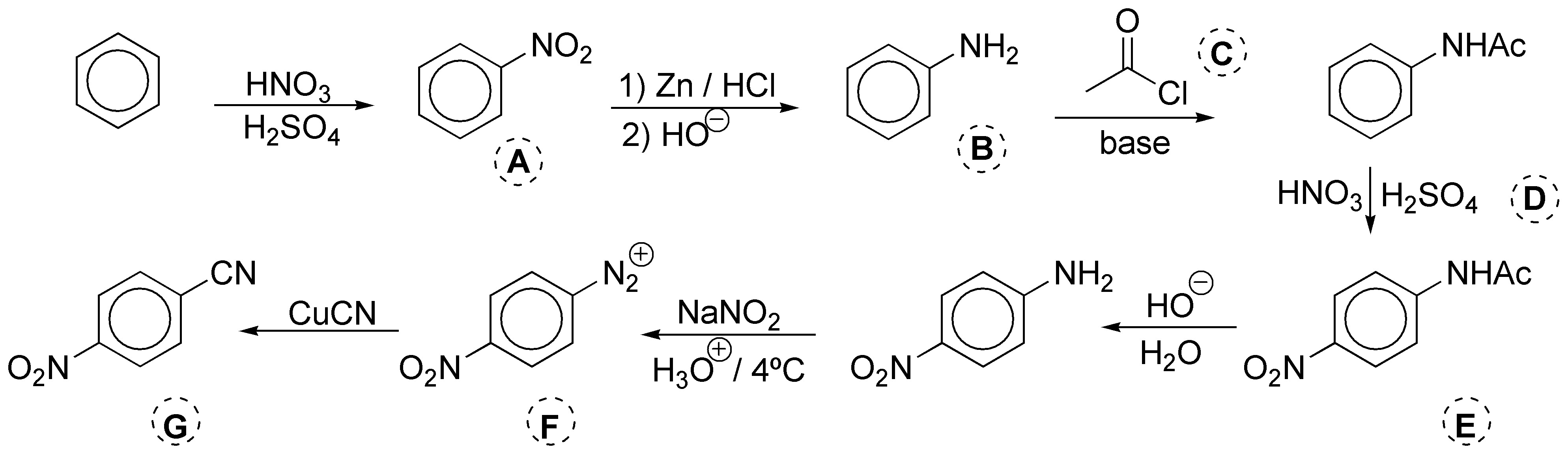
Solution 8:
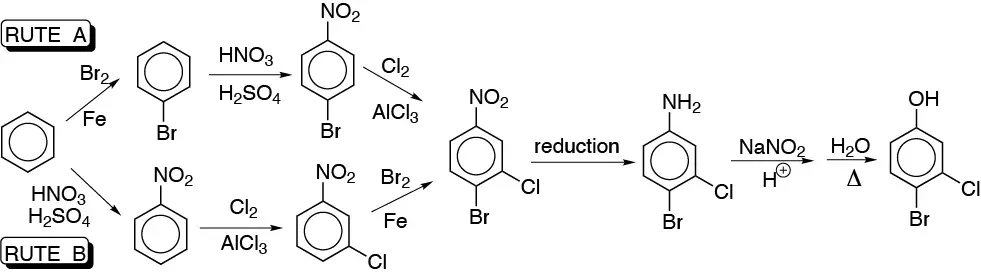
a) Two possible routes can be proposed for the synthesis of this compound, starting from benzene. The number of steps for both routes is the same. The advantage, of route A over route B, lies in the fact that in the first step, Br is weakly deactivating, whereas the nitro group is strongly deactivating, so route A is expected to show a higher overall yield.
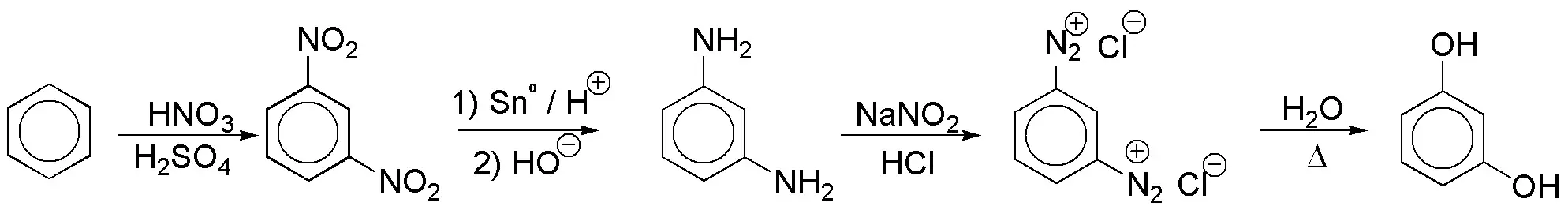
b) The OH groups are located in a relative meta– arrangement so that two groups are required at these positions that can be transformed into OH. The most viable option is to introduce two NO2 groups and transform them into OH by means of diazonium salts. According to this approach the reaction scheme would be:
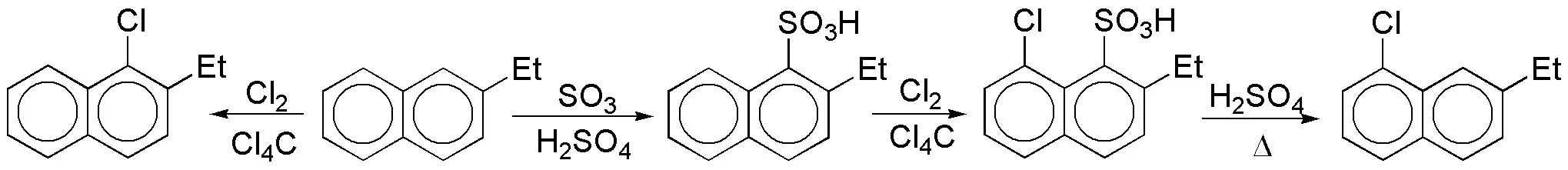
c) Direct halogenation of the starting product does not produce the desired compound, since the most electron-rich ring is the one with the ethyl group. It will be necessary to block the position and make the reactivity of this ring decrease. In this regard, the SO3H group is suitable, since it is deactivating and the sulfonation reaction is reversible.
Solution 9:
a) The key to this first synthesis will be the bromination of the arene and the subsequent nucleophilic substitution on the benzyl bromide obtained with cyanide ion.
In b) since it is impossible to introduce two alkyl residues in meta by successive alkylations, the first step must be a Friedel-Craft acylation, whose subsequent alkylation or acylation will lead to the meta arrangement of the substituents and finally the reduction of the ketone to alkyl.
c) Acylation and subsequent sulfonation leads to the desired product. Not so if the order of the reactions is reversed.
d) Nitration first will direct the subsequent alkylation to the target.
e) Chlorination first (ortho– and para-directing) and subsequent sulfonation.
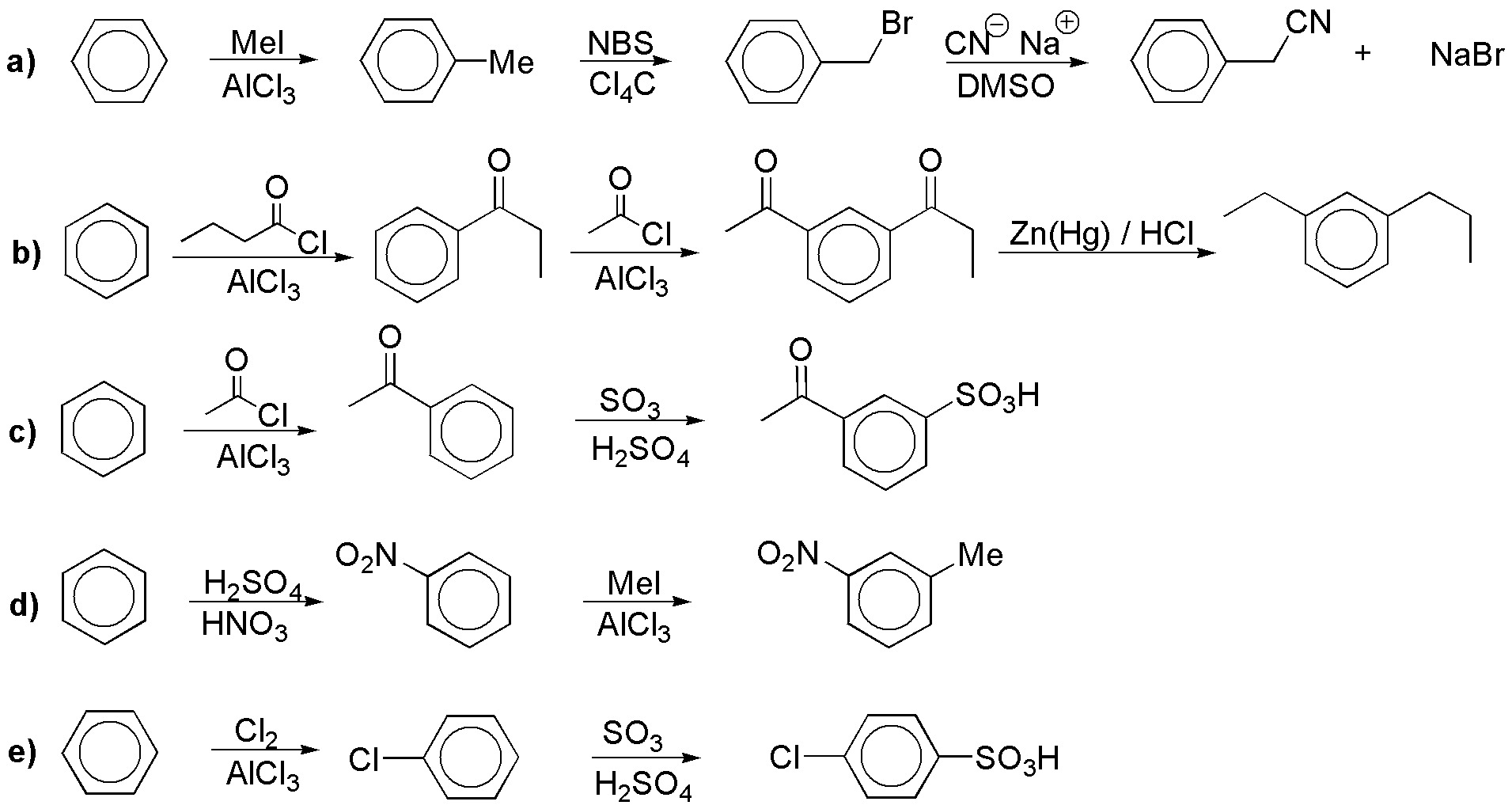
Solution 10:
a) Since fluorine cannot be introduced directly into the aromatic ring, that will be the key to the synthesis. To introduce it we need a diazonium salt (Schiemann reaction). The other key is the introduction of a methyl and an amino in ortho, so we would need to protect the position temporarily.
b) Analogous to the previous one.
c) It is a Friedel-Crafts alkylation.
d) Phenols are usually introduced from diazonium salts.
e) We can introduce chlorine also from diazonium salt (Sandmmeyer reaction).
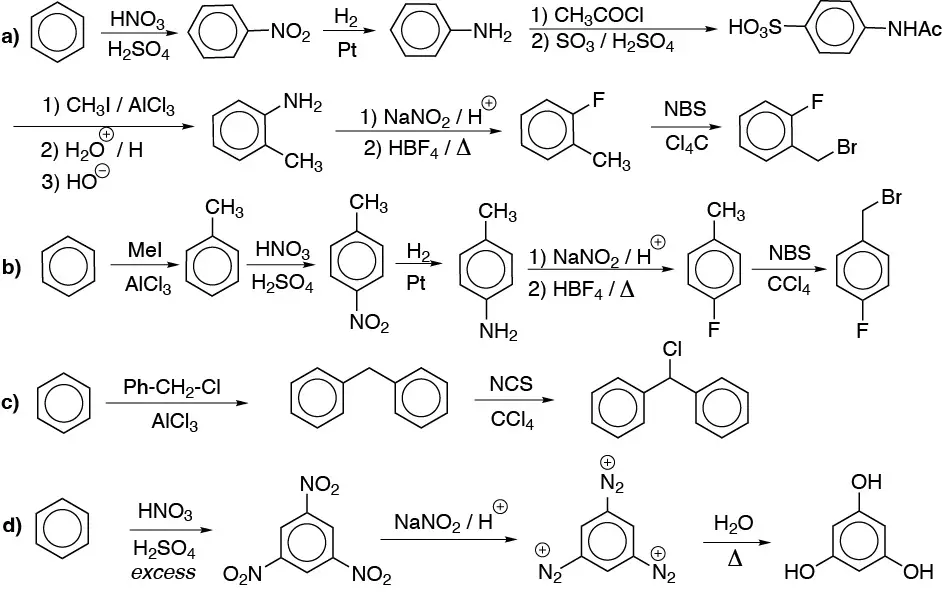
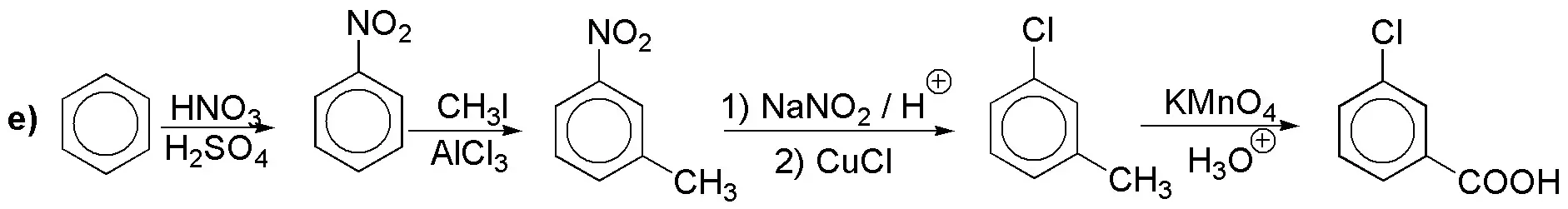
Solution 11:
a) Chlorine and methyl cannot be introduced directly in meta– position. To make the synthesis possible we must do it from a Sandmmeyer reaction with diazonium salts.
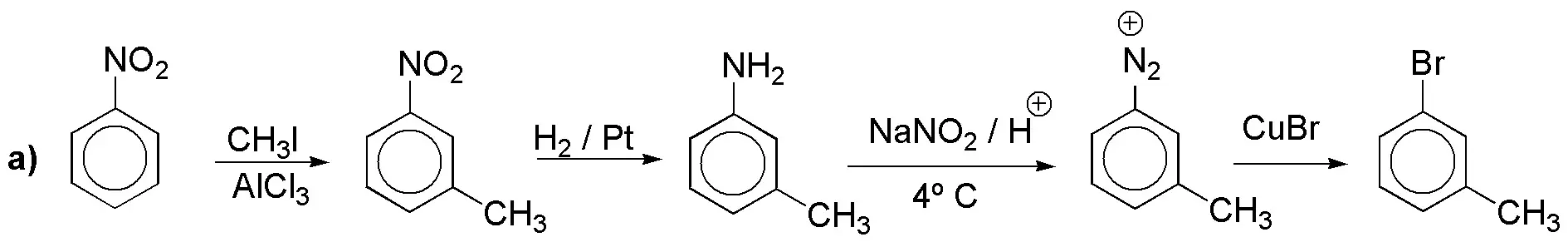
b) The direct nitration of phenol occurs in low yields. Therefore, the introduction of the hydroxyl group will be done from the amino group (diazonium salt).
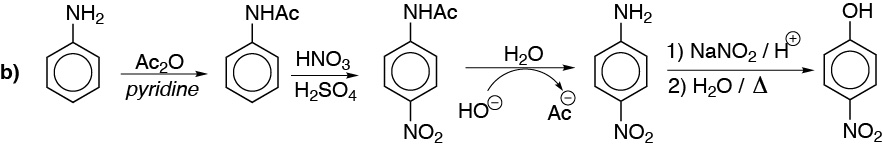
c) With the same considerations as above.
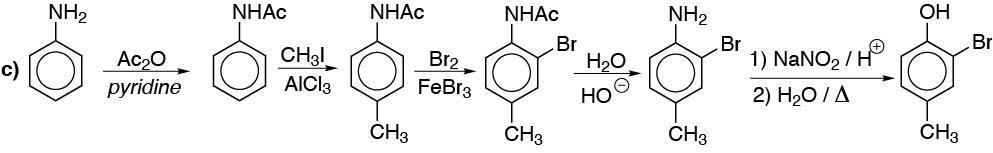
d) Same as in the previous cases, but the arrangement in meta– of the substituents forces us to start from nitrobenzene.
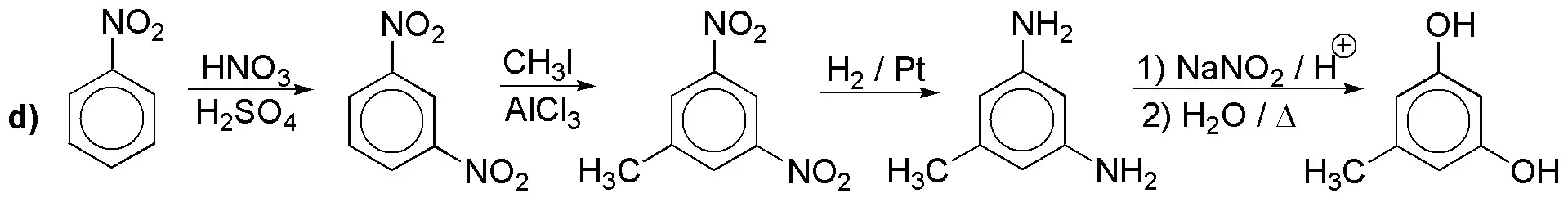
e) Direct introduction of iodine is not possible. The arrangement in meta– forces us to start from nitrobenzene.
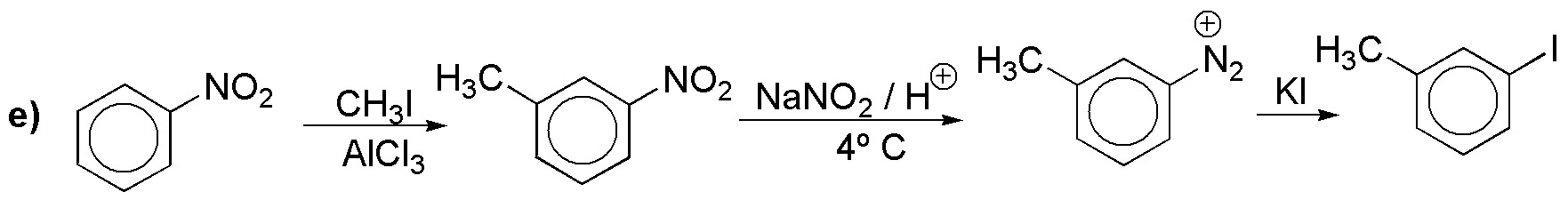
f) It is a compound diazo. We will start from aniline.
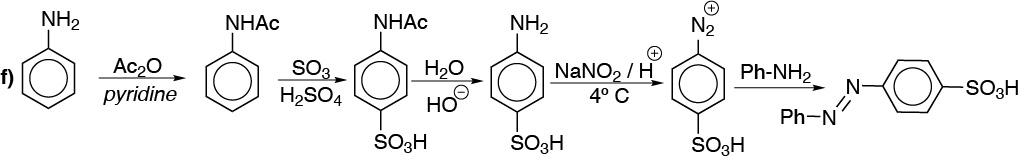
Solution 12:
The solutions proposed are not unique, for all syntheses there are usually several possibilities, here we note some:
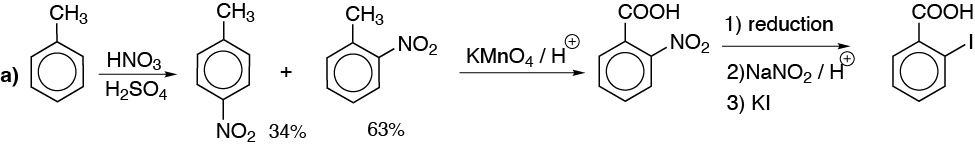
a) The conversion of methyl to carboxylic acid is routine but the introduction of iodine is not. Since it is not possible to introduce an iodine atom into the benzene ring by direct halogenation, we must do it through a diazonium salt.
b) The same diazonium salt as above would serve to obtain the corresponding bromide; another alternative would be the one shown: halogenation followed by oxidation (and it is not the best because the ortho derivative would be in the minority). In problem 18 another synthesis of the same is proposed.
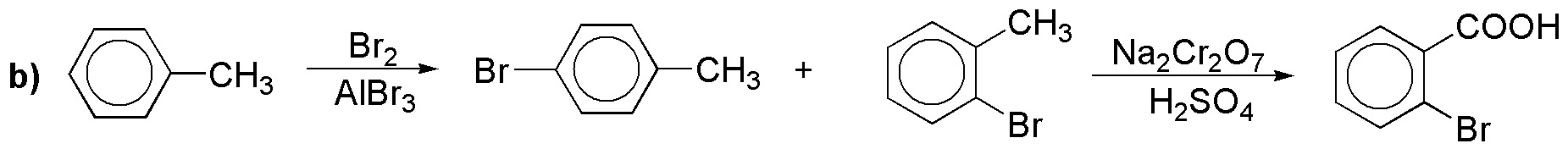
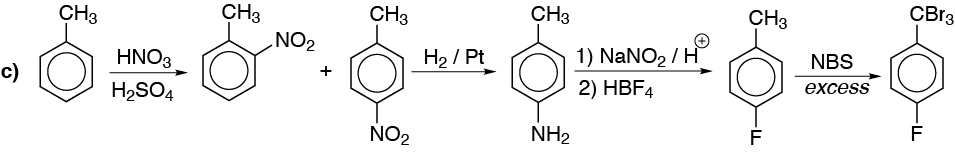
c) As in a) the introduction of fluorine is not a direct reaction of the aromatic ring and we must do it through the diazonium salt. The radical halogenation of methyl can be continued until complete halogenation using excess reagent.
d) The simplest way to introduce an amino into methyl would be by nucleophilic substitution of the corresponding haloderivative:

e) It is not easy to introduce hydroxyls into the aromatic ring. In the laboratory the simplest way is via a diazonium salt:
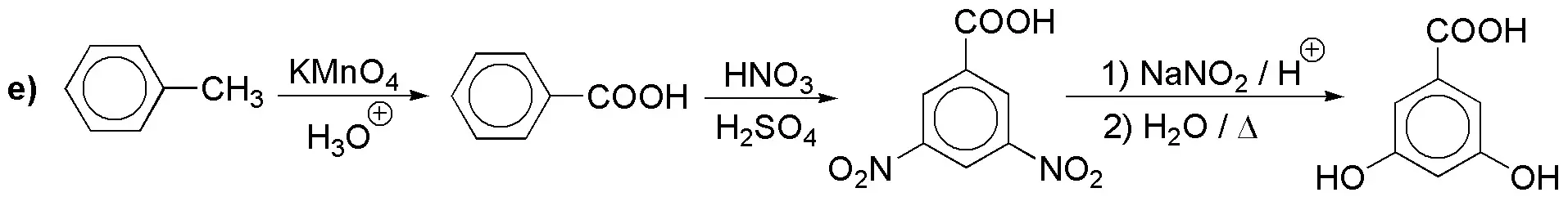
Solution 13:
The neighboring arrangement of the three substituents forces us to temporarily protect the most accessible position 4.
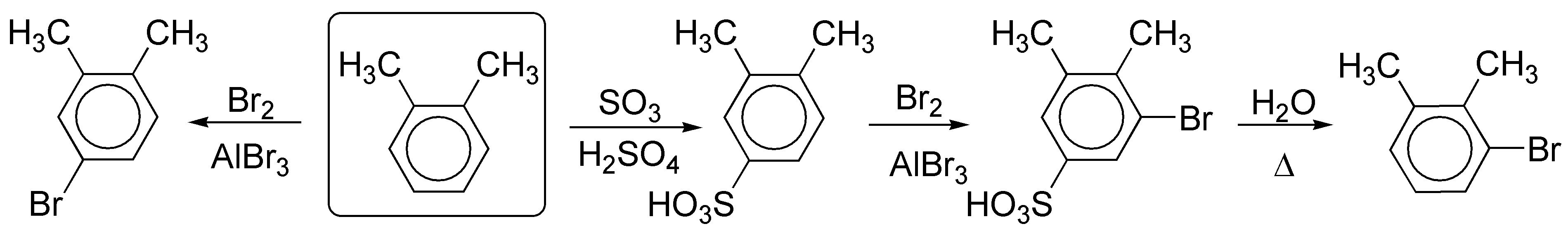
Solution 14:
Methylation and subsequent nitration of benzene leads us to the proper arrangement of the substituents.
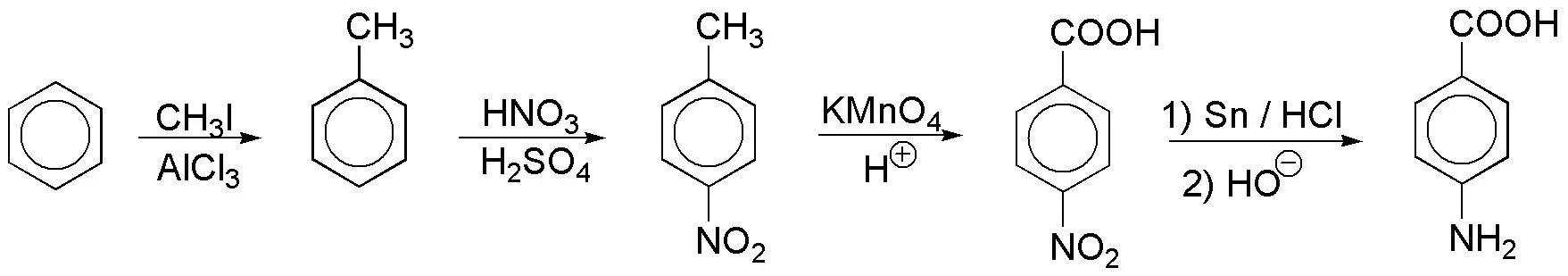
Solution 15:
It is necessary to protect the amino group temporarily in order to use acidic medium. The ortho arrangement of the substituents forces us to also protect the para position with sulfonic acid.
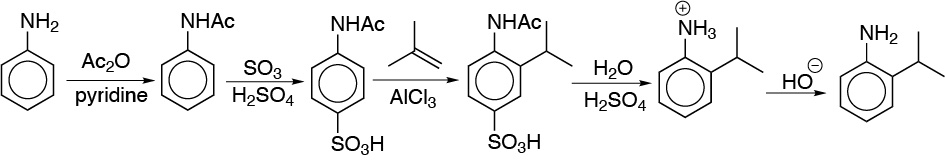
Solution 16:
The same strategy as in the previous problem will be used. To introduce the hydroxyl group we use the diazonium salt.
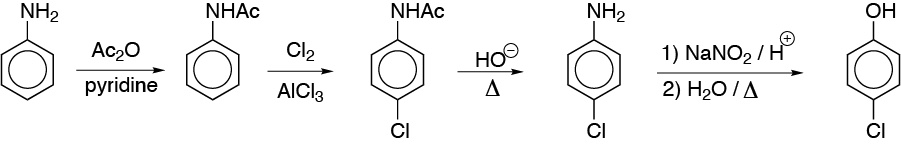
Solution 17:
The keys to this complex synthesis are the two aromatic nucleophilic substitutions indicated in the scheme.
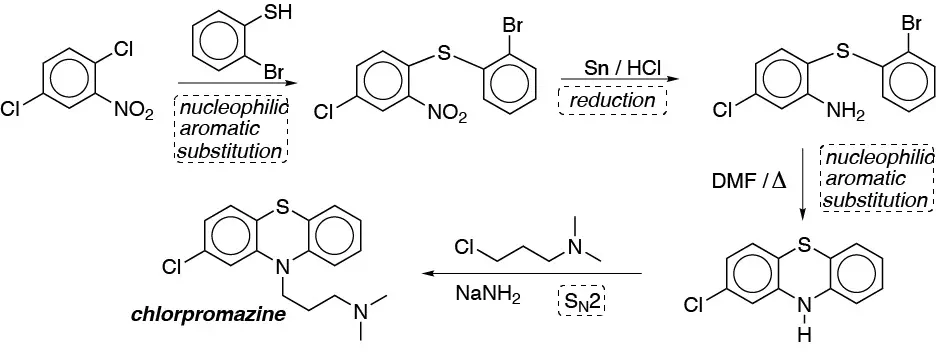
Solution 18:
- a) Since it is not possible to directly introduce the amido group into the aromatic ring, we must introduce the acid by oxidation of the alkyl remainder, subsequently we transform it into the acid chloride and this by reaction with ammonia will produce the amide.
- b) The bromination of the ring and subsequent benzyl halogenation, produces a dihalogenated derivative that by treatment with a base will produce the alkene.
- c) Nitration of the benzene and subsequent chlorination produces the desired meta– arrangement. The nitro group is then reduced.
- d) The key is the formation of the Grignard compound which is nucleophilically added to the cyclohexanone giving the desired product.
- e) The key is the protection of the para position of toluene by sulfonation of toluene, subsequent bromination of the product obtained, methyl oxidation and desulfonation lead to the desired product.
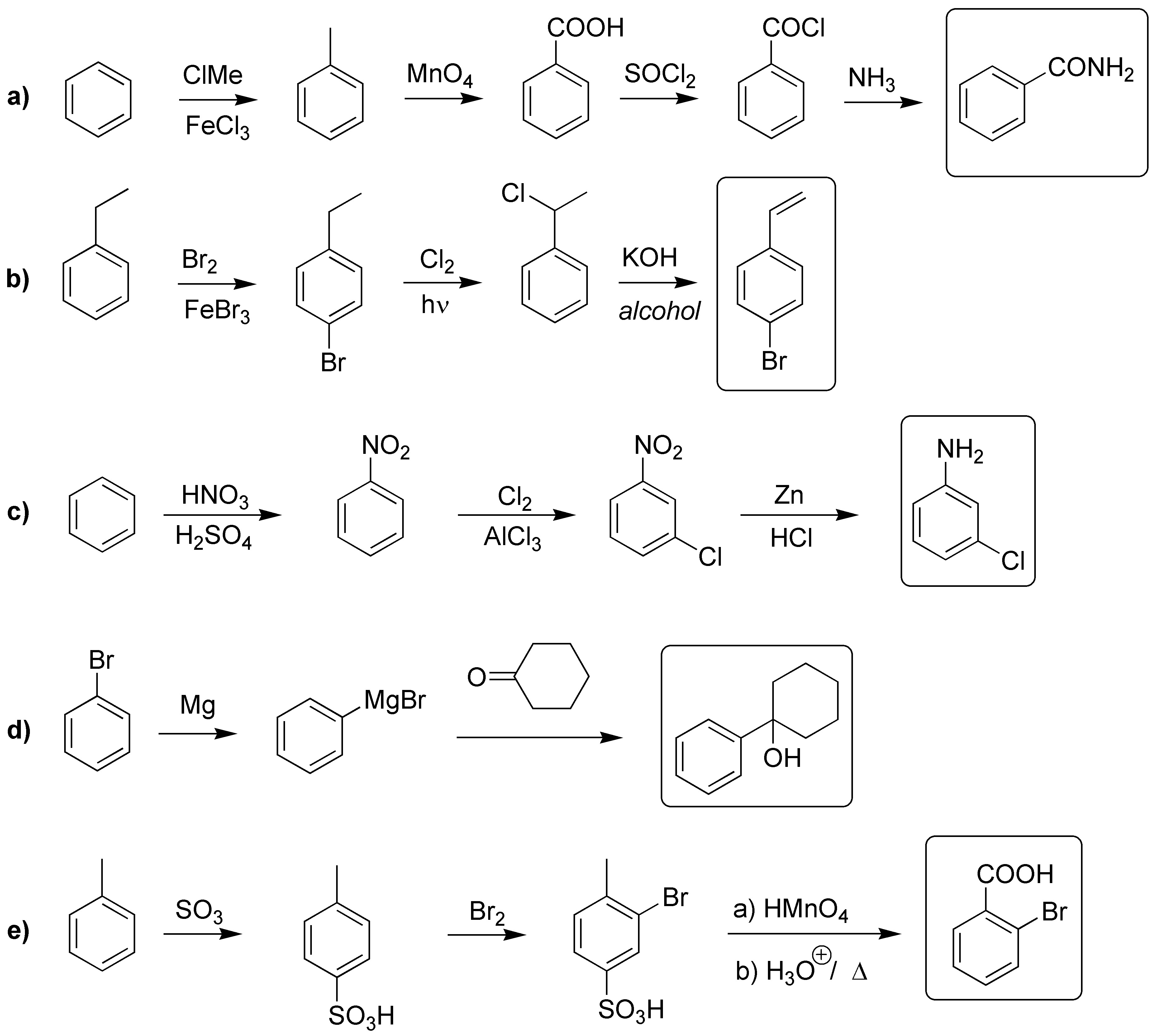
Solution 19:
The methoxyl group will orient in para– giving by acylation the keto acid A. B will be the reduction product of the keto group. Finally C will correspond to the product of a further Friedel-Crafts acylation.
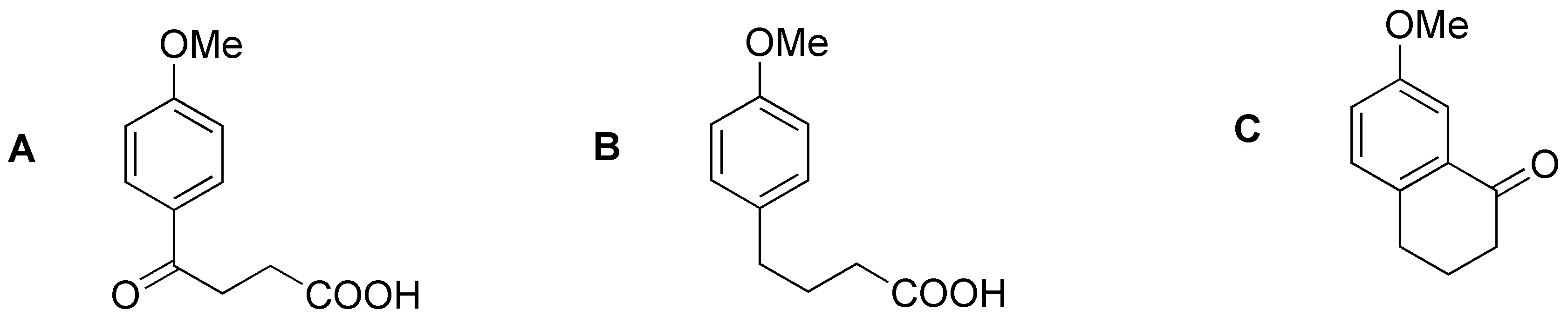
Solution 20:
a) This is a benzyl halogenation. b) The sulfonic groups will be lost. c) and d) are oxidations of alkyl residues to the corresponding carboxylic acids.
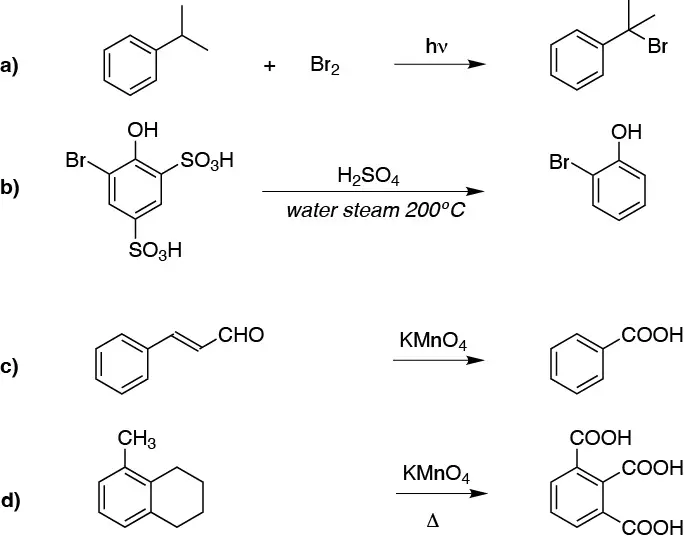
Solution 21:
In principle the greatest difficulty will be in obtaining the ortho-nitrobenzoyl as the sequence is lengthened by the need to protect the para position with respect to the alkyl.
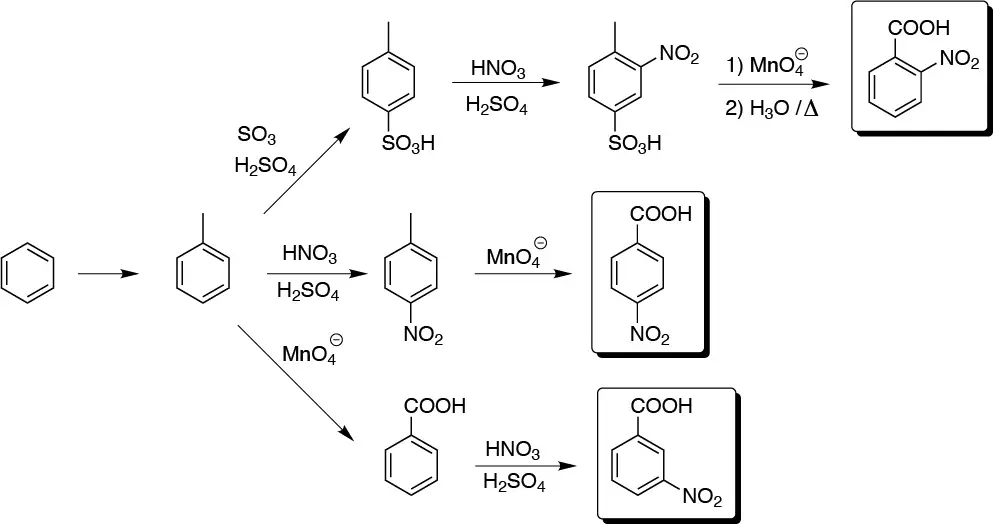
Solution 22:
The electron-attracting groups increase the acidity while the electron-donating ones decrease it therefore the order will be:
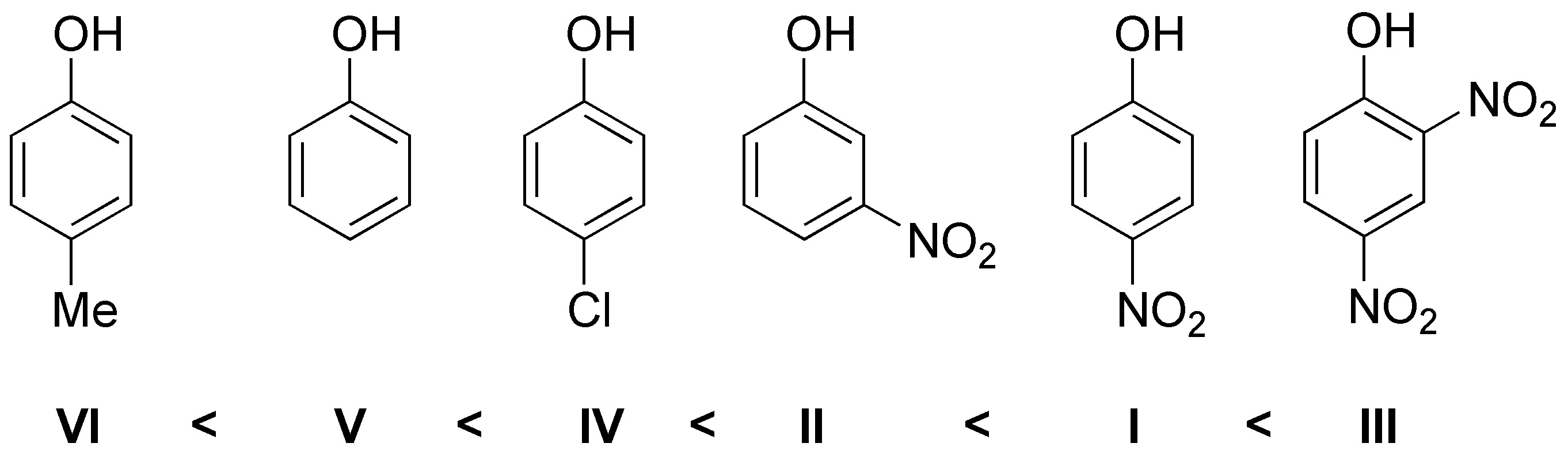
Solution 23:
The most efficient way to introduce the hydroxyl group into the aromatic ring is from the diazonium salt. Therefore, the proposed sequence will be: