Written by J.A Dobado | Last Updated on April 22, 2024

Strontium was discovered in 1791, in an ore found near the Scottish town of Strontia. The ore burned with a bright crimson flame, and the Scottish chemist Thomas Charles Hope studied it and discovered that it contained a new element. This mineral is called strontianite, and it is the main strontium mineral. Thus, in 1808, the chemist Humphry Davy extracted the element from the mineral for the first time, by means of an experiment using electricity. In the past, it was used in television screens, but is less commonly used today. Strontium oxide is used in ceramics and ceramic glazes to create distinctive colors, while strontium carbonate imparts a red color to sparklers and fireworks. Magnets containing iron oxide can be strengthened by the addition of strontium. These powerful magnets are used in loudspeakers and microwave ovens. Strontium chloride is added to some types of toothpaste. On the other hand, radioactive strontium is used as a source of electricity for radar stations in remote locations where there are no power lines or fuel supplies.
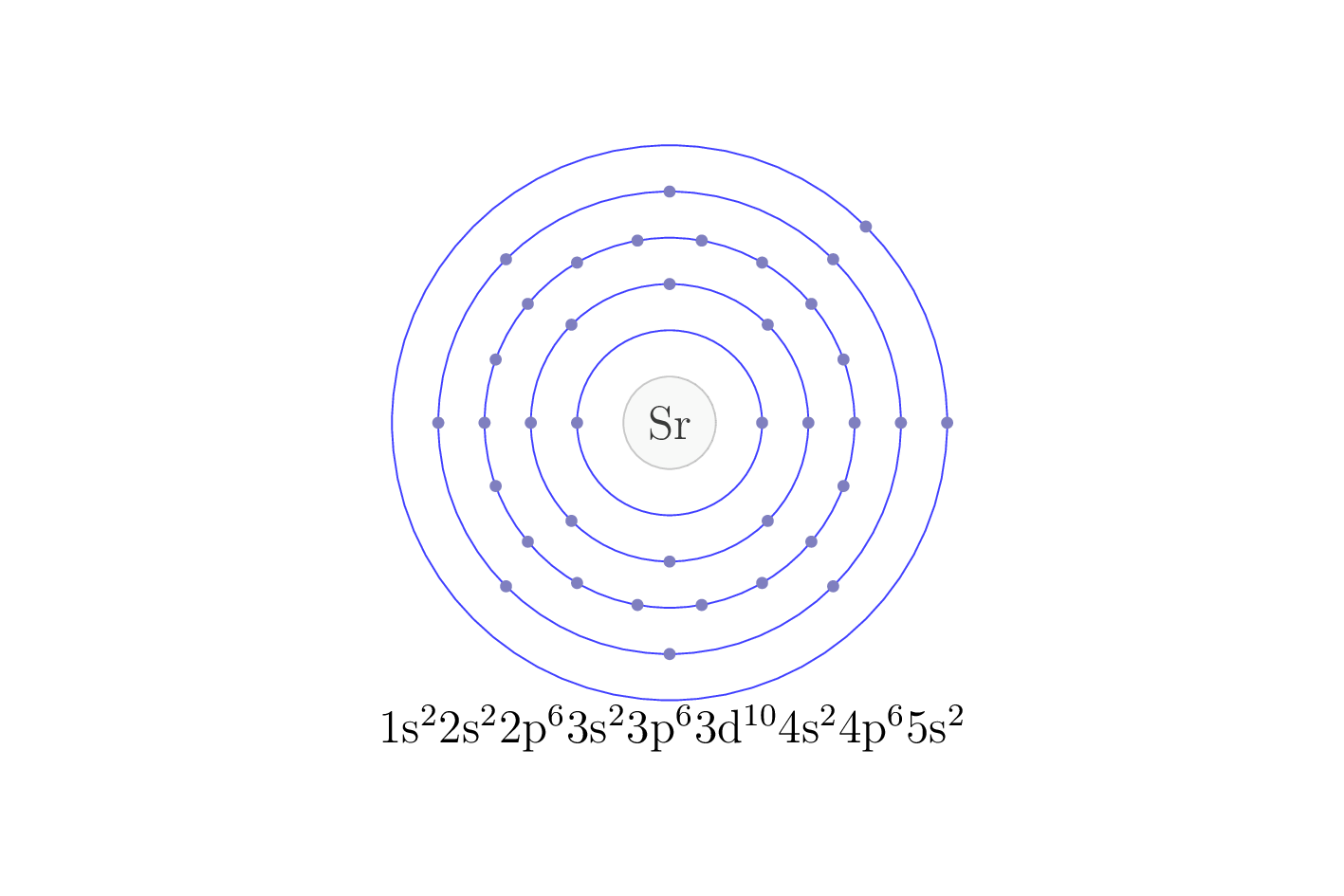
Electron configuration
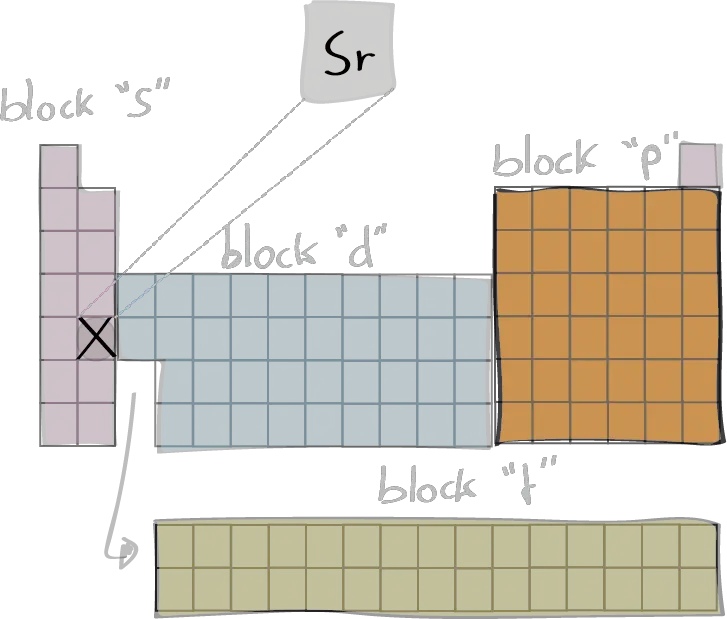
The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its chemical properties and reactivity.
In the electron configuration notation, the letters "s", "p", "d", and "f" represent the different types of atomic orbitals, and the superscripts indicate the number of electrons in each orbital. The orbitals are filled in a specific order, starting with the lowest energy orbital and working up.
Emission spectra
Each element in the periodic table presents its own unique emission spectra, which is determined by the energy levels of its electrons. When an electron in an atom is excited to a higher energy level, it can de-excite by emitting a photon of light with an energy equal to the difference between the two levels. This results in a characteristic emission line in the spectra (which corresponds to specific wavelengths of light). These spectra are usefull to identify the elements present in a sample.

Symmary of properties (Sr)
| Atomic weight | 87.62(1) |
| Discoverer (year) | Crawford, Adair (1790) |
| Natural form | metallic solid (face-centred cubic) |
| Electron configuration | [Kr] 5s2 |
| M.p. (ºC) | 769 |
| B.p. (ºC) | 1384 |
| Earth's crust abundance (ppm) | 370 |
| Isotope (abundance %) | 84Sr (0.56), 86Sr (9.86), 87Sr (7.00), 88Sr (82.58) |
| Density (g/cm3) | 2.54 |
| vdW radius (pm) | 249 |
| Covalent radius (pm) | 190 |
| Electronegativity (Pauling) | 0.95 |
| Vaporisation enthalpy (Kj/mol) | 139.00 |
| Fusion enthalpy (kJ/mol) | 7.43 |
| Specific heat capacity (J/g·K) at 25ºC and 1 at | 0.30 |
| Thermal conductivity (W/cm·K) at 25 ºC and 1 at | 0.350 |
| Oxidation number | 2 |
| Electronic affinity (eV) | 0.05 |
| 1st Ionization energy (eV) | 5.6949 |
Definition of terms in the previous table
- Atomic weight: The average mass of an element's atoms, typically given in atomic mass units (amu).
- Natural form: The most stable and abundant form of an element that occurs naturally in the environment.
- Electron configuration: The arrangement of electrons in an atom or molecule.
- Melting point: The temperature at which a solid substance turns into a liquid.
- Boiling point: The temperature at which a liquid substance turns into a gas.
- Earth's crust abundance (ppm): The concentration of an element in the Earth's crust, typically given in parts per million (ppm).
- Isotope (abundance %): A variant of an element that has the same number of protons in the nucleus, but a different number of neutrons. The abundance of an isotope is the percentage of the isotope in a sample of the element.
- Density (g/cm3): The mass of a substance per unit volume.
- vdW radius (pm): The radius of an atom or molecule as predicted by the van der Waals model, typically given in picometers (pm).
- Covalent radius (pm): The distance from the center of an atom to the center of another atom with which it is bonded covalently, typically given in picometers (pm).
- Electronegativity (Pauling): A measure of an atom's ability to attract electrons in a chemical bond, based on the Pauling scale.
- Vaporisation enthalpy (kJ/mol): The amount of energy required to convert a substance from a liquid to a gas at a constant temperature.
- Fusion enthalpy (kJ/mol): The amount of energy required to convert a substance from a solid to a liquid at a constant temperature.
- Specific heat capacity (J/g·K) at 25ºC and 1 at: The amount of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius at a constant pressure.
- Thermal conductivity (W/cm·K) at 25 ºC and 1 at: The ability of a substance to conduct heat, typically given in watts per centimeter per kelvin.
- Oxidation number: A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound.
- Electronic affinity: The energy change associated with adding an electron to a neutral atom to form a negative ion.
- 1st Ionization energy: The energy required to remove the most loosely bound electron from a neutral atom.