What is Boyland-Sims oxidation?
The Boyland-Sims Oxidation, also referred to as the Boyland-Sims peroxydisulfate oxidation, was initially discovered by Boyland and Sims in 1953. This particular oxidation reaction converts primary, secondary, and tertiary aromatic amines to o-sulfate esters, which are then further transformed into the corresponding alcohols. The Boyland-Sims Oxidation is a two-step process that first involves the oxidation of aromatic amines by alkaline persulfate, yielding predominantly the o-amino aryl sulfates. The second step involves the conversion of o-amino aryl sulfates into o-hydroxy aryl amines through acid-promoted hydrolysis.
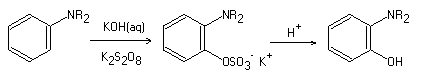
Mechanism of reaction
According to the proposed reaction mechanism, the reaction begins with an ipso attack by peroxydisulphate. This is followed by the cleavage of the peroxide bond, resulting in the formation of an ortho-sulphate ester, as depicted in the figure below:
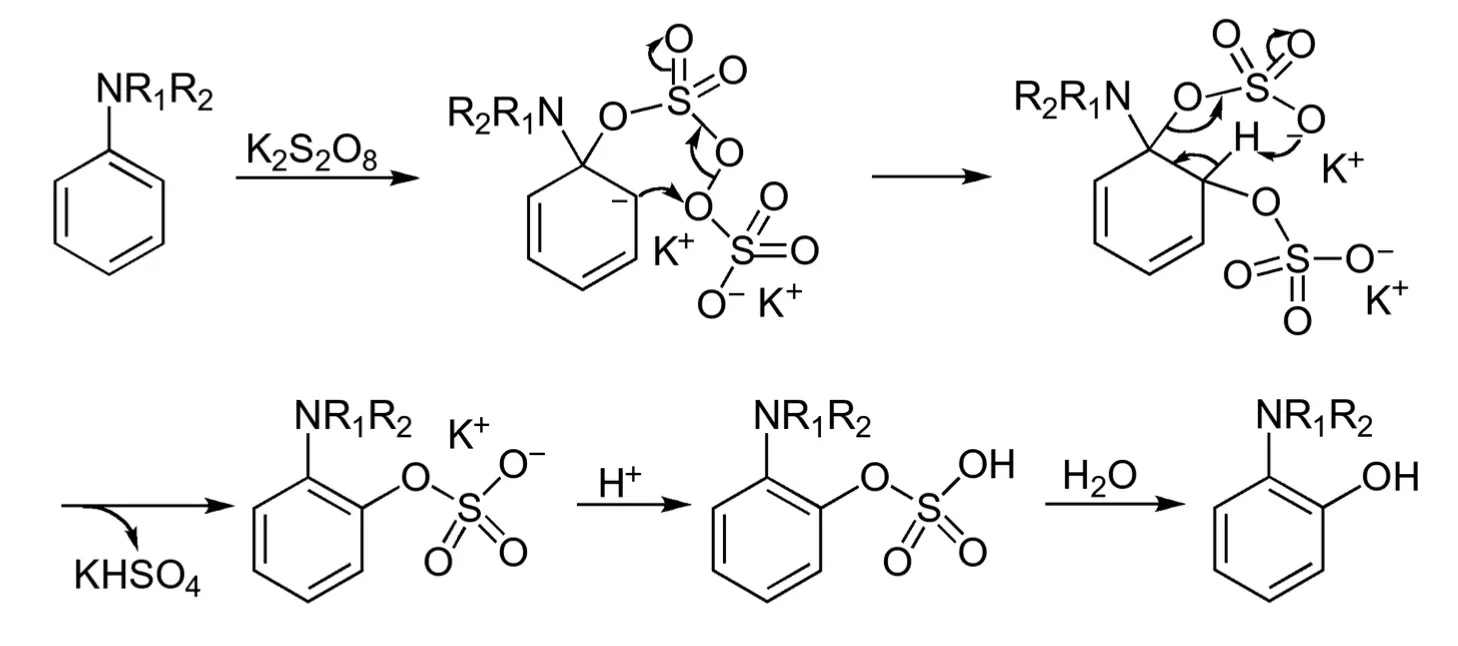
References
Boyland, E.; Manson, D.; Sims, P., “The preparation of o-aminophenyl sulphates” J. Chem. Soc., 1953, 3623-3628
DOI: 10.1039/JR9530003623
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.