What is Hofmann-Martius rearrangement?
The Hofmann-Martius rearrangement, or aniline rearrangement, which involves the thermal conversion of hydrohalides of N-alkylated aromatic amines to produce o-alkylated and p-alkylated aromatic amine derivatives, was initially documented by Hofmann and Martius in 1871.
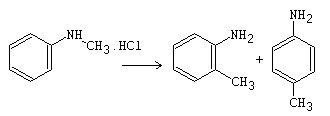
When N-alkylated aromatic amine hydrochlorides are heated, they dissociate, and the resulting components undergo intermolecular alkylation through the migration of an alkyl group in the form of a free radical, olefin, or carbocation. Evidence suggests that both radical and olefin pathways may be involved. In the solid state, the Hofmann-Martius rearrangement primarily produces ortho-alkylated products, likely due to restricted diffusion. Additionally, the rearrangement can be initiated by photo-irradiation.
References
- Hofmann, A.W. and Martius, C.A. (1871), Methylirung der Phenylgruppe im Anilin. [Methylation of the phenyl group in aniline.] Ber. Dtsch. Chem. Ges., 4: 742-748. https://doi.org/10.1002/cber.18710040271
- Hofmann, A.W. (1872), Umwandlung des Anilins in Toluidin. [Conversion of aniline to toluidine.] Ber. Dtsch. Chem. Ges., 5: 720-722. https://doi.org/10.1002/cber.18720050241
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.