What is Sugasawa reaction?
The Sugasawa reaction is a chemical process that involves the acylation in the ortho position of anilines using nitriles in the presence of BCl3 and an auxiliary Lewis acid.
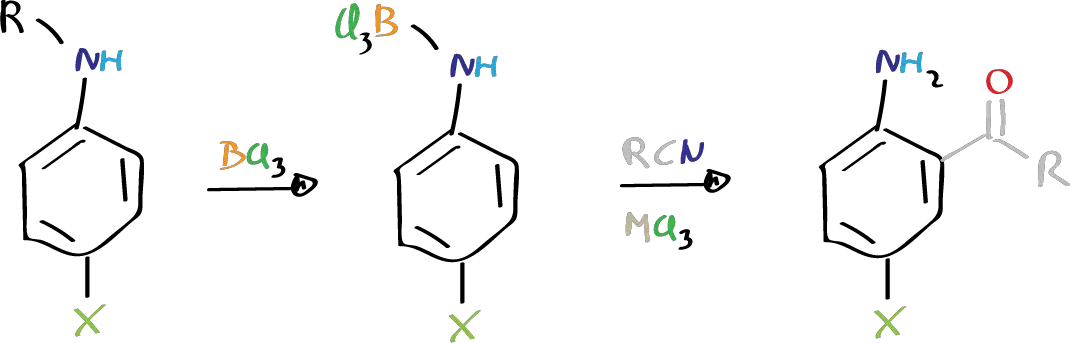
X = 2nd Lewis acid
References
- Aminohaloborane in organic synthesis. 1. Specific ortho substitution reaction of anilines
Tsutomu Sugasawa, Tatsuo Toyoda, Makoto Adachi, and Kazuyuki Sasakura
Journal of the American Chemical Society 1978 100 (15), 4842-4852
DOI: 10.1021/ja00483a034 - Adachi, Makoto; Sasakura, Kazuyuki; Sugasawa, Tsutomu, “Aminohaloborane in Organic Synthesis. IX. Exclusive ortho Acylation Reaction of N-Monoaminoalkylanilines” Chemical and Pharmaceutical Bulletin 33, 1826-1835 (1985)
DOI: 10.1248/cpb.33.1826
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.