What is Stevens rearrangement?
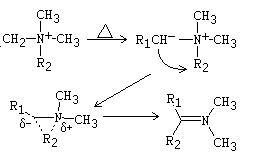
The Stevens rearrangement is a chemical process that involves the migration of an alkyl group from a sulfonium or quaternary ammonium salt to an adjacent carbanionic center upon treatment with a strong base. This reaction ultimately leads to the formation of a rearranged tertiary amine or sulfide compound as the end product.
References
- T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “CCCCXXIII.—Degradation of quaternary ammonium salts. Part I” J. Chem. Soc. 1928, 3193
DOI: 10.1039/JR9280003193 - T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “CCLXX.—Degradation of quaternary ammonium salts. Part II” J. Chem. Soc. 1930, 2107
DOI: 10.1039/JR9300002107 - T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “CCLXXI.—Degradation of quaternary ammonium salts. Part III” J. Chem. Soc. 1930, 2119
DOI: 10.1039/JR9300002119 - T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “7. Degradation of quaternary ammonium salts. Part IV. Relative migratory velocities of substituted benzyl radicals” J. Chem. Soc. 1932, 55
DOI: 10.1039/JR9320000055 - T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “262. Degradation of quaternary ammonium salts. Part VI. Effect of substitution on velocity of intramolecular rearrangement” J. Chem. Soc. 1932, 1926
DOI: 10.1039/JR9320001926 - T.S. Stevens, E.M. Creighton, A.B. Gordon, M. MacNicol, “263. Degradation of quaternary ammonium salts. Part VII. New cases of radical migration” J. Chem. Soc. 1932, 1932
DOI: 10.1039/JR9320001932
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.