What is Overman rearrangement?
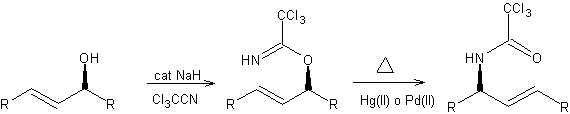
The Overman rearrangement is a formal [3,3]-sigmatropic rearrangement of the trichloroacetimidate of allylic alcohols to allylic trichloroacetamides. Thus, transposing the hydroxyl and amino functions with chirality transfer.
References
- Overman, L. E. “Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions” J. Am. Chem. Soc.1974, 96, 597−599. DOI: 10.1021/ja00809a054
- Overman, L. E. “A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions” J. Am. Chem. Soc.1976, 98, 2901−2910. DOI: 10.1021/ja00426a038
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.