What is dienone-phenol rearrangement?
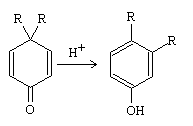
The dienone-phenol rearrangement is a chemical reaction in which a cyclohexadienone molecule with substituents at positions 4 and 4 undergoes transformation into a phenol molecule with substituents at positions 3 and 4, upon exposure to an acidic environment.
References
- v. Auwers, K. and Ziegler, K. (1921), Über Kohlenwasserstoffe der Semibenzolgruppe. [On hydrocarbons of the semibenzole group.] Justus Liebigs Ann. Chem., 425: 217-280. https://doi.org/10.1002/jlac.19214250302
- Inhoffen, H.H., Huang-Minlon (1938) Übergang von Sterinen in aromatische Verbindungen. III. Mitteilung: Aromatisierung des Δ1, 2; 4, 5-Cholestadienons-3. [Transition of sterols into aromatic compounds. III. communication: Aromatization of the Δ1, 2; 4, 5-cholestadienone-3.] Naturwissenschaften 26, 756
DOI: 10.1007/BF01774198 - Inhoffen, H.H., Stoeck, G. and Lübcke, E. (1949), Totalsynthese des 1′,6-Dimethyl-1,2-benzanthracens und des 1′,5,6-Trimethyl-1,2-benzanthracens. [Total synthesis of 1′,6-dimethyl-1,2-benzanthracene and 1′,5,6-trimethyl-1,2-benzanthracene.] Justus Liebigs Ann. Chem., 563: 177-185. https://doi.org/10.1002/jlac.19495630202
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.