What is Meyer-Schuster rearrangement?
The Meyer-Schuster rearrangement is a reaction that is catalyzed by acid and involves the rearrangement of secondary and tertiary α-acetylenic alcohols to α,β-unsaturated carbonyl compounds. The Meyer-Schuster rearrangement leads to the formation of aldehydes when the acetylenic group is terminal, and ketones when it is internal.
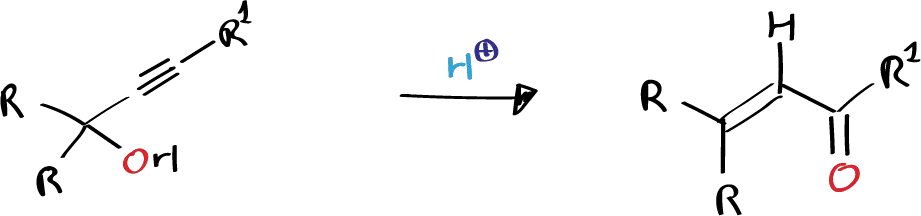
In 1926, a rearrangement closely related to the Meyer-Schuster rearrangement was described and named the Rupe rearrangement. The Rupe rearrangement involves the conversion of tertiary alkylacetylenic carbinols with a terminal acetylenic group into predominantly α,β-unsaturated ketones, rather than the expected aldehydes.
References
- Meyer, K.H. and Schuster, K. (1922), Umlagerung tertiärer Äthinyl-carbinole in ungesättigte Ketone. [Rearrangement of tertiary ethynyl carbinols to unsaturated ketones.] Ber. Dtsch. Chem. Ges. A/B, 55: 819-823. https://doi.org/10.1002/cber.19220550403
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.