Objective
To extract essential oils from cinnamon and/or cloves by hot extraction using an organic solvent under reflux and then removing the solvent under reduced pressure (rotary evaporator).
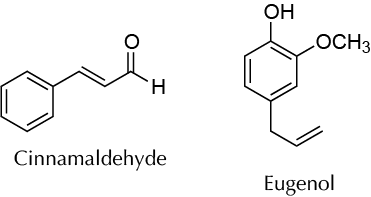
Background
Many plants are commonly used for their pleasant fragrances or flavors caused by the presence of certain molecules that provide them with their organoleptic properties. The use of plants to give food flavor and aroma is a practice known since antiquity. Cinnamon, cloves, garlic, mint, and vanilla are some common examples of this experiment as rooted in different cultures and are also fundamental elements of the human diet that enrich food and make it attractive. On the other hand, the use of fragrances and essences for healing or for ornamental purposes has also been known for thousands of years. Essential oil is a concentrated substance, usually volatile, extracted from a plant. Essential oils are generally located in the seeds or flowers but may exist in other plant parts such as stems, leaves, or roots. The term “essential” comes from the belief that the oil represented the essence of the odor or taste of a particular plant species. Essential oils are used in almost all types of food production, such as the manufacture of candies, pastries, pickles, drinks, and perfume. They can be isolated from a plant by various processes or a combination of processes, such as mechanical pressing, grinding, maceration, solvent extraction, or distillation.
Procedure
Weigh 5.0 g of spice (cinnamon or clove), and finely grind using a mortar. Transfer the solid to a 100 ml round-bottom flask, add 35 ml of ethyl acetate, and use a stirbar (or boiling chip). Fit a condenser, reflux the mixture for 30 min. Cool the flask contents to r.t. and filter by gravity into a dry Erlenmeyer with a fluted paper filter. Wash the round-bottom flask with 10 ml of ethyl acetate, and pour the solvent onto the solid remaining in the fluted paper filter to ensure that all the extract from the species is obtained. Then dry the ethyl acetate solution over anhydrous sodium sulfate, and filter by gravity into a 100 ml tared round-bottom flask. Remove the solvent under reduced pressure (rotary evaporator), weigh the contents, and determine the percentage of essential oil from the starting sample. For cinnamon, the main essential oil component will be cinnamaldehyde, while for cloves it will be eugenol. Alternatively, the two phases can be separated by decanting in a separatory funnel, but this is less effective.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Cinnamaldehyde | 132.16 | -7.5 | 248 | 1.050 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| Eugenol | 164.20 | -7.5 | 254 | 1.067 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Cinnamaldehyde |  |
| Ethyl acetate |   |
| Eugenol |   |
| Na2SO4 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Cinnamaldehyde | KJPRLNWUNMBNBZ-QPJJXVBHSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| Eugenol | RRAFCDWBNXTKKO-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
Back to the Basic Laboratory Operations experiments page.
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.