What is a Chemical Reaction?
The definition of a chemical reaction is very simple. Chemical bonds between atoms are broken and new bonds are formed. Two types of substances are involved in this process: those that we initially have and know as reactants and those that are obtained after the chemical reaction, called products.
Speed of Reaction
The rate of a reaction is the change that you experience the concentration of a reactant or product over time, and is expressed with the following equation:
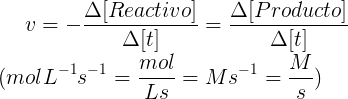
You must take into account the minus sign if this is the speed at which it is consumed as a reagent and in the case of a product will be positive, since it refers to the speed at which such product.
The general equation that describes the rate of a reaction would be:
aA +bB → cC + dD
where a, b, c, and d are the stoichiometric coefficients of the reaction.
You can calculate the average speed of the reaction with any of the components in the following way:
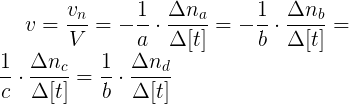
The average speed should always be positive. If the reaction takes place at constant volume, for example, when species are all dissolved, the equation can be expressed using concentrations:
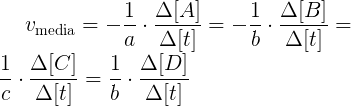
Reactive nucleophile and electrophile
Reactive nucleophilic (or nucleophile): species capable of donating electrons (base Lewis acid), because it presents pairs of electrons without sharing, negative charge, or multiple bonds.
Reactive electrophilic (or electrophile): species capable of accepting electrons (acid Lewis acid), due to which has a positive charge, or formal charge positive, with orbital empty capable of accepting electrons.
Keep in mind that the concepts of acid/base Lewis acid are related to equilibrium (thermodynamic point of view) while reactive nucleophile/electrophile these concepts are kinetic.
Breaking and formation of bonds
In organic compounds the reactions take place by breaking and formation of bonds. The links may be broken, or form in one of two ways:
Homolytic bond breaking: occurs when a covalent bond breaks in such a way that each atom retains one electron, generating normally radicals (chemical species with an odd number of electrons).
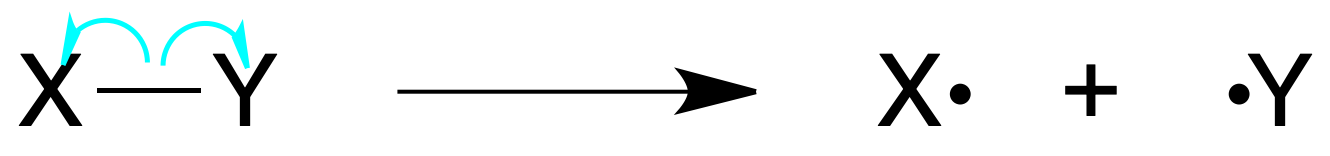
Also, the formation of bonds from radicals is produced by reacting two radical species that bring every one of them an electron to the bond.
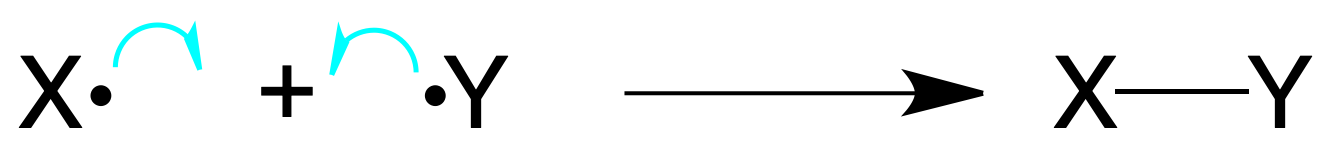
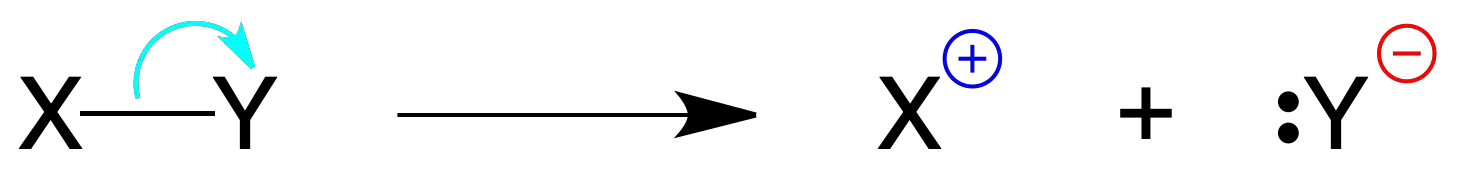
Heterolitic bond breacking: when the breakage of the bond is not symmetrical, one of the fragments retains the two electrons of the covalent bond.
This process originates, usually, a species negatively charged (which retains the two electrons) and one positively charged (indicated in the figure by ⊕ and ⊖).
Also, the formation of links from ions it is produced by reacting two charged species, one of them bringing the pair of electrons and the other with positive charge.
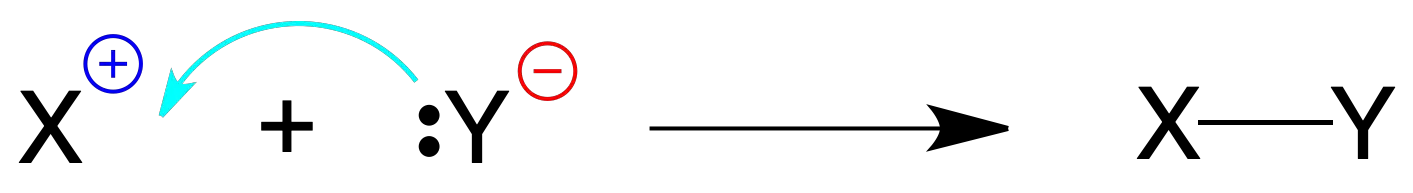
Arrows curved: it is used in reaction mechanisms to show the flow of electrons. This type of arrows curved is used only for this purpose. The origin of the arrow is associated with the electrons and the tip with the destination of the same.
We use two types of arrows (indicated in color in figures): a double-tip to indicate the movement of a pair of electrons, commonly associated with the processes with breaking and formation of bonds heterolítica, and another arrow with a single tip (type hook) to show the movement of a single electron when there is breaking or forming bond homolytic (for example, reactions, radical, radical formation, etc).
Types of chemical reactions
A chemical reaction it can be defined as the process of interaction between chemical species that, as a consequence of the breaking and formation of bonds, it creates a new chemical entity.
The organic reactions are classified according to different criteria, resulting in the six types of reactions are shown below:
- Addition reaction: two or more molecules combine to form a larger one.
For example:
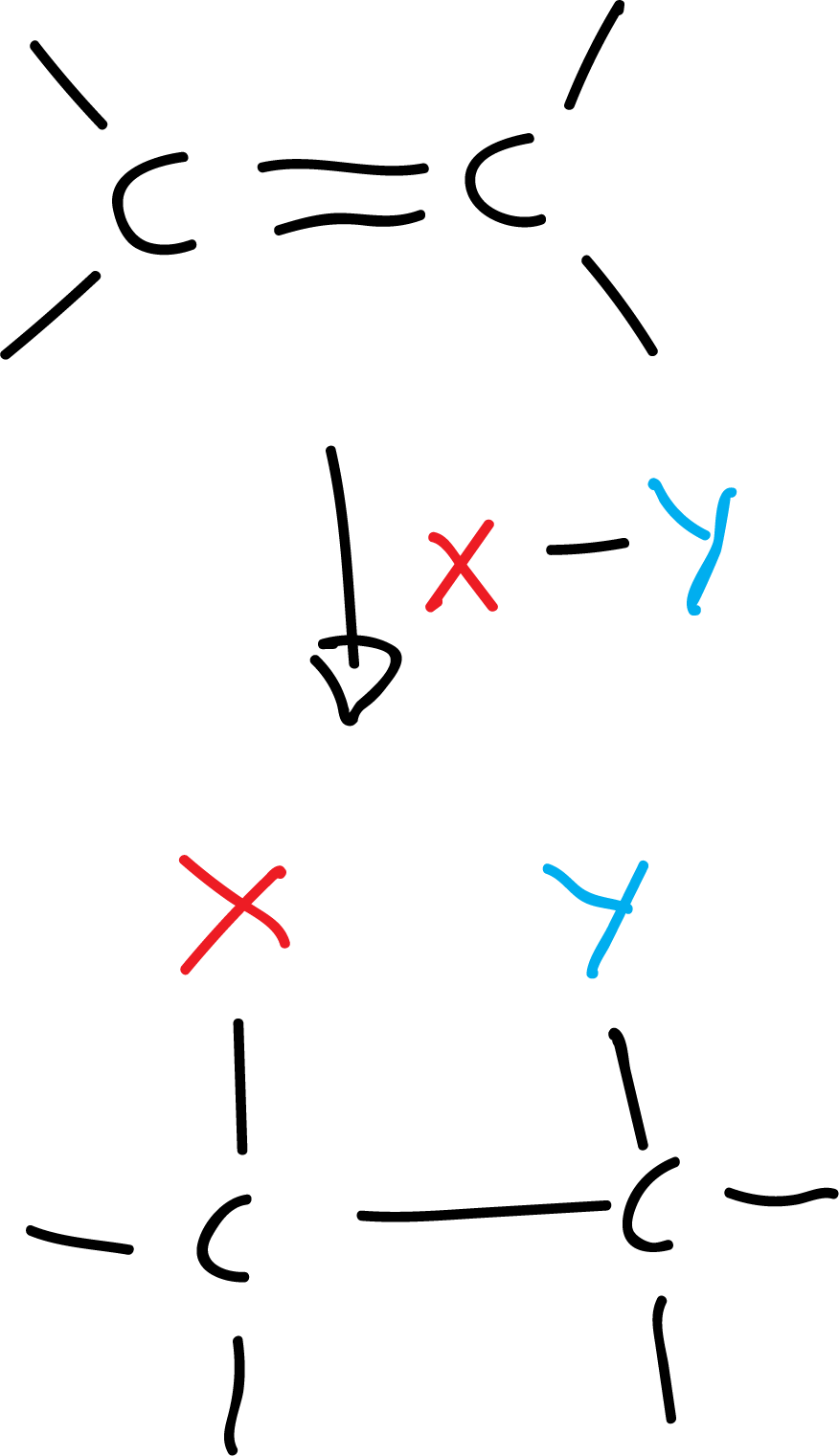
There are three types of addition reactions (nucleophilic, electrophilic and radical).
- Reaction of removal: it is the inverse of the addition reaction and involves the loss of an atom (or group of atoms) of a molecule, to form bonds or multiple rings. It can occur in one or two stages. For example:
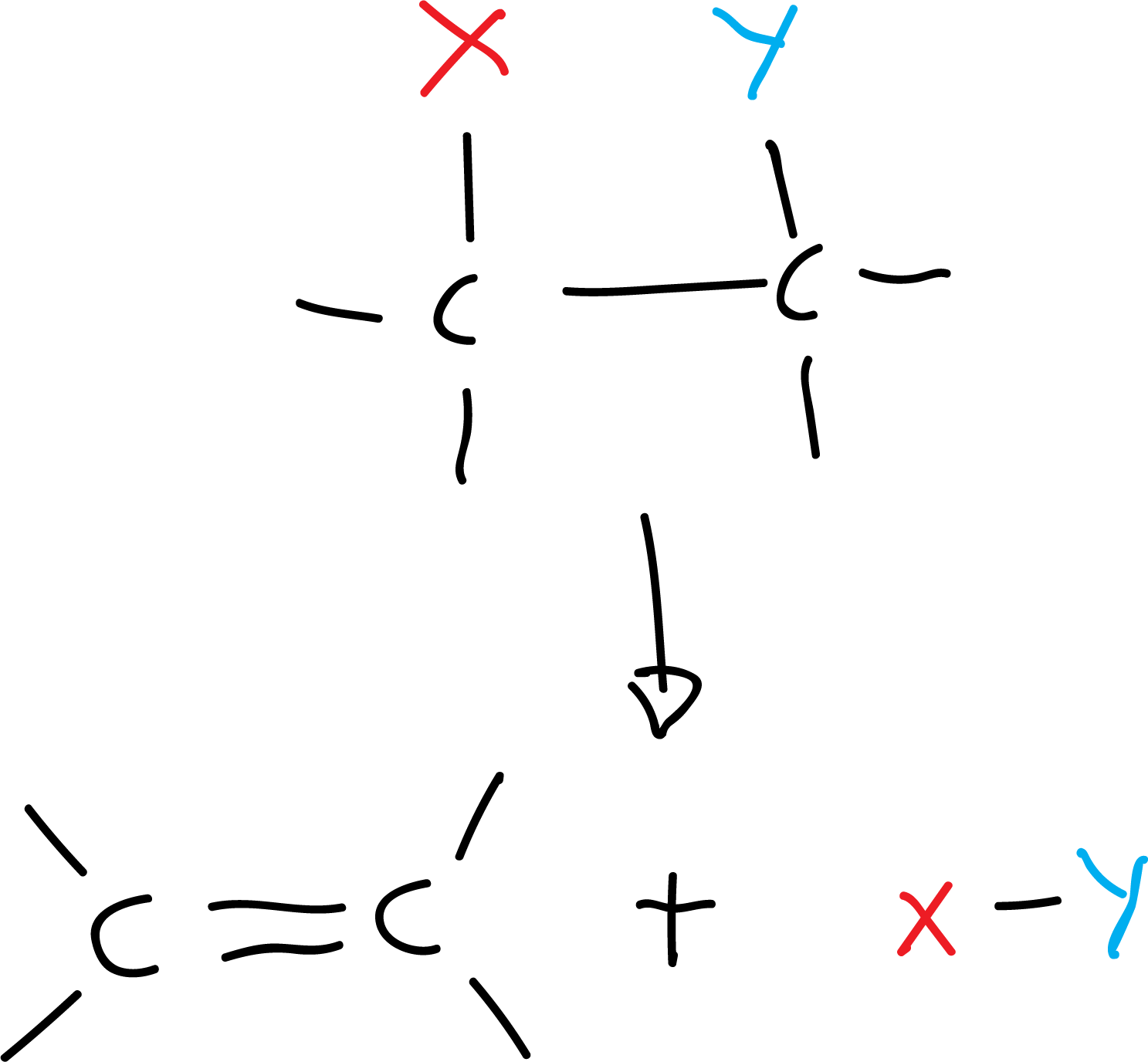
- Substitution reaction: it consists in the replacement of an atom (or group of atoms) on the other. For example:
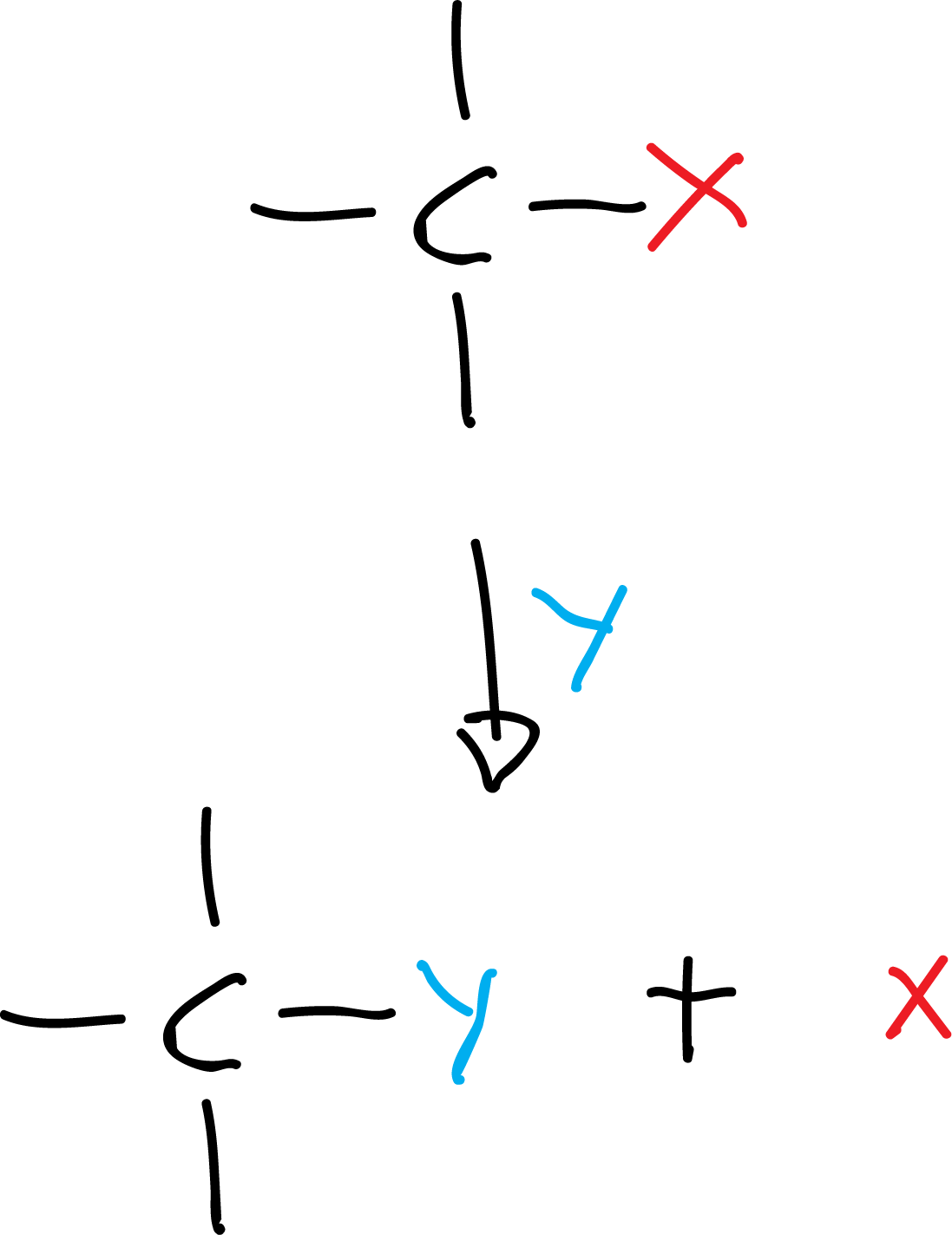
There are three types of substitution reactions (nucleophilic, electrophilic and radical). The following figures illustrates an example of a substitution reaction radical and its mechanism:
fig-7
- Regrouping or transposition: are rearrangements of the skeleton of a molecule to give rise to a structural isomer of the molecule of departure. For example:
fig-8
- Pericyclic reactions: take a concerted manner (the bonds are broken and formed at the same time) through a state of transition cyclic.
fig-9
- Redox reactions (oxidation and reduction): Are reactions in which there is a change in the oxidation numbers of some atoms in the reactants. Are not, in the strictest sense, a new kind of reactions, but rather changes that may accompany other types of reactions are described. For example:
fig-10
The oxidation reactions can be identified in many cases, the gain of oxygen and halogens and/or the loss of hydrogen in the molecule of the game, while the reduction reactions tend to be associated with the loss of oxygen or halogen, and/or the gain of hydrogen on the substrate of departure (see oxidation number).
Yield of a chemical reaction
Prior to calculating the yield of a reaction is necessary to know the stoichiometry (the setting of the chemical reaction so that there is the same number and type of atoms in the reactants than in the products), which presents the reaction and to know what is the limiting reagent (the one expressed in mol is in a smaller amount, according to the stoichiometry).
Yield of a single-reaction: the amount of product obtained in a chemical reaction. Is expressed as relative performance (in percent, %), and results from dividing the mol obtained product between mol theoretical product(maximum amount that could be obtained from product if reaccionase the entire amount of the limiting reagent). For your calculation, you must take into account the limiting reagent and the stoichiometry of the reaction.
A → B
Efficiency (%) = [(mol obtained product) / (mol theoretical product)]×100
Yield of a multistep reaction: the overall performance of a transformation that takes place with the many chemical reactions are calculated by multiplying the partial yields (expressed as a percentage of one) of each of the reactions that comprise it.
A → B → C → D → etc
Overall yield (%) = (performance 1st reaction × performance 2nd reaction × performance 3rd reaction, … etc.) × 100
For example: a chemical transformation consists of 3 reactions and the partial yields of each one of them are 25%, 50% and 75% to calculate the overall performance is applied to the above equation (expressing the partial yields as a percentage of one) and we obtain:
Overall yield (%) = 0,25 × 0,50 × 0,75 × = 9,4%
Video about types of Chemical Reactions
Thermodynamics and chemical kinetics
The thermodynamic study basically, the energy changes associated with the processes of equilibrium and its evolution in chemical reactions.
Equilibrium constant (Keq): governs the concentrations of reactants and products at equilibrium. For example:
fig-11
From the Keq you can calculate the free energy change (ΔG) of a reaction. Is expressed by the equation (ΔG = Gproducts – Greagents). Typically, you use the free energy change of Gibbs (ΔGº), when reactants and products are in their standard states (at 25 ºC and 1 atm). The relationship between ΔGº and Keq is given by the following equation (ΔGº = –RT log Keq), where R= 1,99·10-3 kcal·kelvin-1·mol-1; T = absolute temperature in kelvin. RT 25 ° C = 0,593 kcal· mol-1.
The reactions with high values of Keq favoured, if they have ΔGº negative (energy is released). However, if there are positive values of ΔGº they will be disadvantaged and therefore, it is necessary to supply energy to the system to have such a reaction. In addition, ΔGº is a function of the enthalpy (ΔHº), which is the waste heat or consumed in a chemical reaction, the entropy (ΔSº), which measures the freedom of movement of the system, and the temperature (T) according to the equation:
ΔGº = ΔHº – T·ΔSº
Normally, the enthalpy governs the chemical reaction, with the contribution of the change of entropy for the temperature despicable. The change of enthalpy is a measure of the strength of the bonds of the reactants and products. Reactions tend to favor products with a lower enthalpy (stronger bonds). If broken bonds are weaker and are other more strong releases heat in the reaction, and says that it is exothermic (ΔHº negative). However, if you break the stronger bonds and are the weakest, then it consumes energy to be the reaction endothermic (ΔHº positive).
You can calculate, approximately, the value of the enthalpy of reaction from tabular values of the bond dissociation energy (see Table 1). When they form bonds is apparent energy, while breaking it requires energy.
| Bond | ΔHº |
| H-H | 104 |
| H-F | 138 |
| H-Cl | 103 |
| H-Br | 88 |
| CH3-H | 105 |
| CH3-F | 110 |
| CH3-Cl | 85 |
| CH3-Br | 71 |
| CH3CH2-H | 98 |
| CH3CH2–F | 107 |
| CH3CH2–Cl | 80 |
| CH3CH2–Br | 68 |
| CH3CH2CH2-H | 98 |
| CH3CH2CH2–F | 107 |
| CH3CH2CH2–Cl | 81 |
| CH3CH2CH2–Br | 68 |
| (CH3)2CH2-H | 95 |
| (CH3)2CH2–F | 106 |
| (CH3)2CH2–Cl | 81 |
| (CH3)2CH2–Br | 68 |
| (CH3)3C-H | 93 |
| (CH3)3C-F | 110 |
| (CH3)3C-Cl | 81 |
| (CH3)3C-Br | 67 |
| F-F | 38 |
| Cl-Cl | 58 |
| Br-Br | 46 |
Example: the enthalpy of fluorination of the ethane is calculated from the values of the bond dissociation energies of the following form:
fig-12
count the bonds that are formed or broken in the process, in this case a F-F bond broken (+38) and other C-H (+98) to form one H-F (-138) and other C-F (-107). Therefore, the total balance of enthalpy is calculated in the following way: ΔHº =(+38+98) + (-138-107) = +136 + (-245) = –109 kcal·mol-1 and the reaction is exothermic.
Kinetics studies the rate of chemical reactions. The rate of a reaction is determined by measuring the concentrations of products and reactants through the time. The way in which is dependent on the concentrations of reactants and products is called speed equation. Each reaction has its own rate equation to determine experimentally.
For example: in the fluorination reaction of ethane the speed of the reaction expressed by:
v = k [CH3CH3]·[F2]
it is said that it is of first order with respect to each of the reactants, because it is proportional to the first power of concentration. In addition, it is of second-order general because it is the sum of the two powers of the concentrations. This equation is to determine experimental-mind and cannot be predicted from the stoichiometry of the reaction, although sometimes match.
Arrhenius equation expresses the dependence of the rate constant (characteristic of each reaction) with the reaction conditions.
K = A·e–Ea/RT
where, A is a constant. (pre-exponential factor), Ea it is the activation energy, R is the gas constant 1,99·10-3kcal·kelvin-1·mol-1 and T the absolute temperature.
The above concepts are best understood graphically using diagrams of reaction that relate to the energy that you put in the game in a chemical process for going from the reactants to the products, to some extent, indicating that the reaction takes place (variations in the concentration, bond length, etc):
fig-13
In the figure above, a generic energy profile is illustrated for two reactions, one exothermic and one endothermic. The vertical axis indicates the total potential energy of all chemical species involved in the reaction. The horizontal axis is the reaction coordinate and represents the progress of the reaction, on the left would be the reactants and on the right the products. The transition state (TS) is the maximum point of the graph, and is, logically, between the reactants and the products. The activation energy (Ea) is the energy difference between the transition state and the reactants. ΔHº is the difference between the energy of the reactants and the products.
Hammond–Leffer postulate says that in the profile of energy for a chemical reaction exothermic, the transition state will be close to the reactants (structures and energies similar). On the contrary, for reactions endothermic, the transition state will be next to the products.
Intermediate of a chemical reaction
Are species with a half-life time is very short, and that are not present in high concentrations, due to rapidly evolving. Graphically, are identified as the minimum relative energy in the reaction coordinate.
In Organic Chemistry, the reaction intermediates most common are species with carbon trivalent and according to its load are called:
fig-15
Carbocations: chemical species, positively-charged (the carbon with the positive charge has all the valence electrons forming bonds). The stability increases with the degree of substitution of the carbon trigonal, because the alkyl groups give slightly electrons, thereby stabilizing the carbocatión (inductive effect).
Present a structure trigonal planar in the carbon hybridization sp2.
fig-16
Radical (or free radicals): chemical species neutral, in which the carbon is trivalent presents an electron without sharing. The carbon presents hybridization sp2with an electron without sharing in an orbital p. Like the carbocationes is a species deficient in electrons and the stability increases with the degree of substitution.
fig-17
Carbanions: chemical species with a negative charge, in which the carbon atom trivalent presents a pair of electrons without sharing. Unlike the previous cases, the carbon presents a hybridization sp3, with a pyramid shape of the substituents.
fig-18
Carbene complexes: chemical species without cargo but with a carbon atom divalent, which has two electrons without sharing. The simplest of the species is :CH2 and is called methylene. The carbon presents a hybridization sp2; depending on how they are situated electrons can speak of methylene singlet or triplet.
fig-19