What is nucleophilic substitution (SN1 and SN2)?
Nucleophilic substitution (SN1 and SN2) is probably one of the most versatile reactions in Organic Synthesis, since it allows to obtain a great variety of functions.
The general scheme of the reaction is as follows:
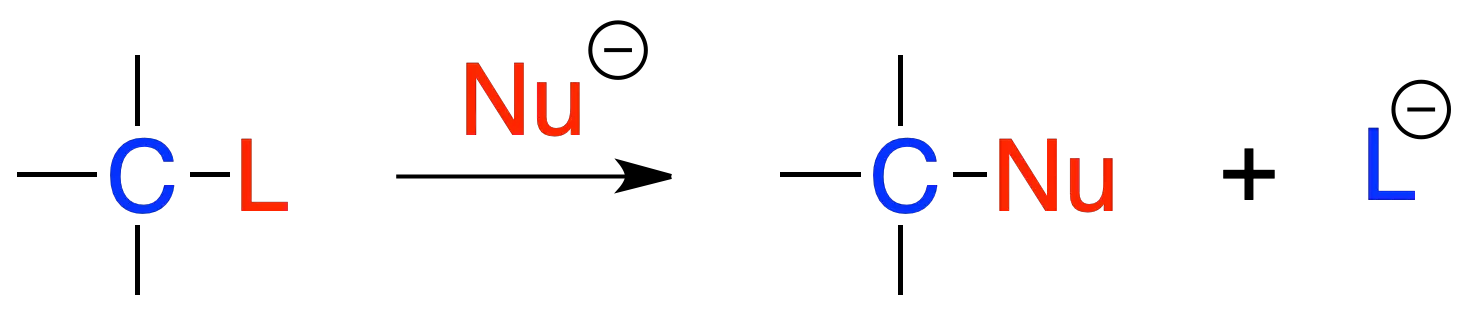
where L is the so-called leaving group and Nu a nucleophile. The C–L bond is broken and a new C–Nu bond is formed. The reaction takes place according to two mechanisms:
Bimolecular nucleophilic substitution SN2
In the SN2 reaction, the C–Nu and C–L bonds are formed and broken simultaneously as shown in the following scheme:
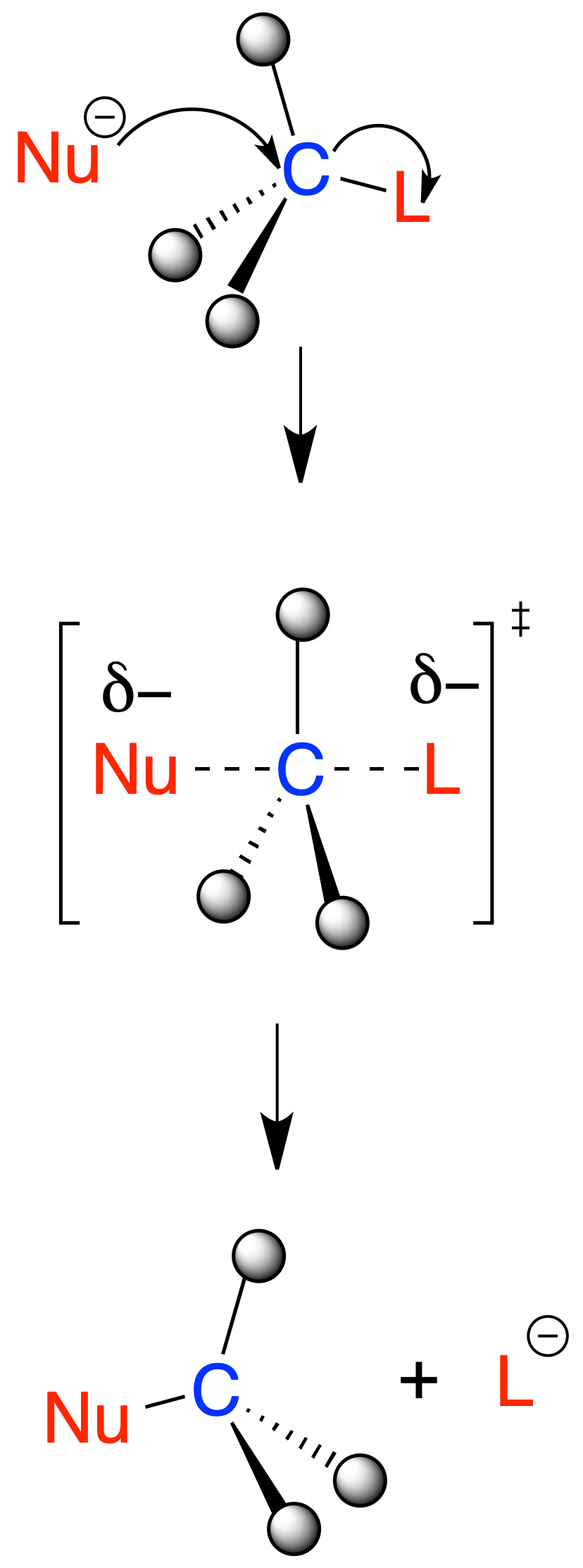
It has second-order kinetics (v = k·[sustrato]·[nucleófilo]) and proceeds with configuration inversion through a pentacoordinate transition state.
Unimolecular nucleophilic substitution SN1
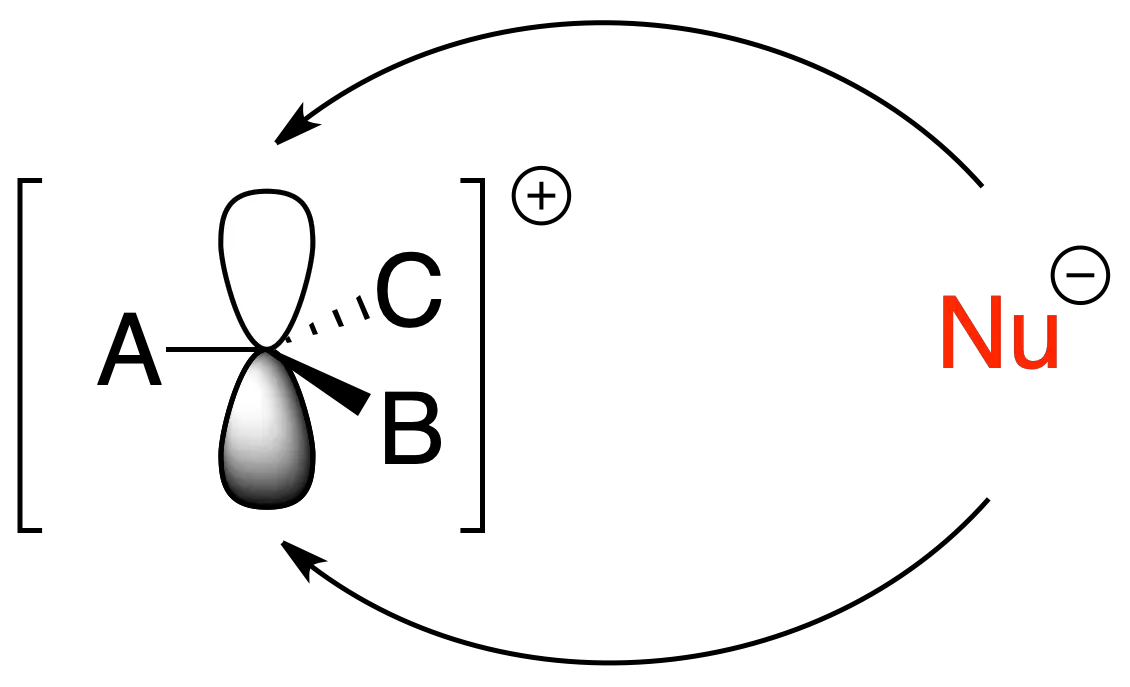
In the SN1 reaction, the C–L bond is broken, a carbocation is formed and the nucleophile attacks it to give a C–Nu bond. It has first-order kinetics (v = k·[sustrato]) and proceeds with racemization when the starting substrate is chiral. When the reaction is solvent-driven, it is called solvolysis.
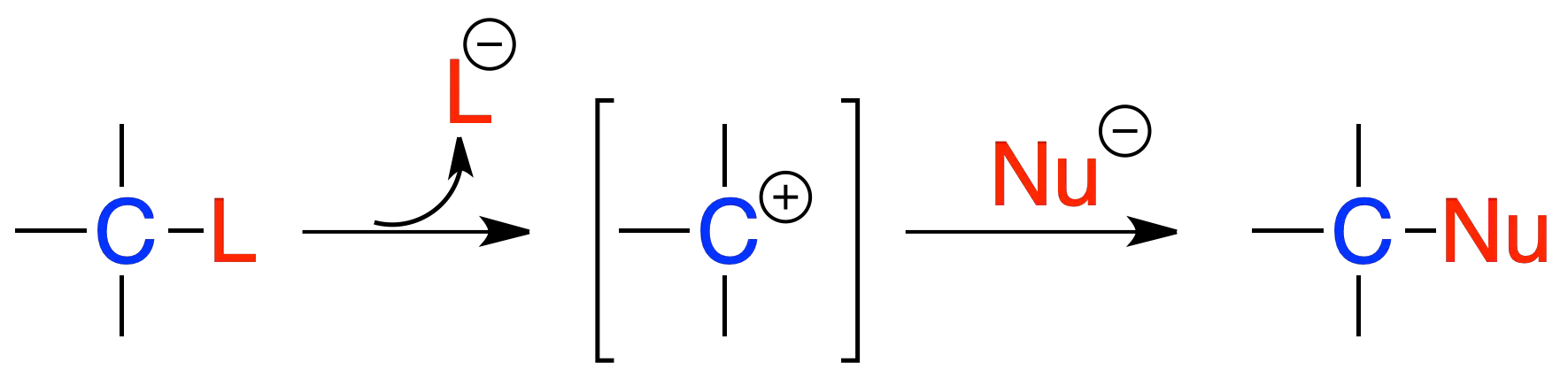
Factors affecting nucleophilic substitution (SN1 and SN2)
The mechanism of a substitution reaction can be predicted quite accurately by taking into account the factors that affect the development of this reaction:
a) Substrate b) Leaving group c) Nucleophile d) Solvent
Effect of substrate on the reaction
The nature of the substrate is one of the most relevant factors in the nucleophilic substitution reaction. The table shows the behavior of different substrates and their tendency to give or not to give substitution reactions by a SN1 or SN2mechanism.
| Substrate | SN1 | SN2 |
| CH3-X | Never in dissolution | Rapid with good nucleophiles |
| R-CH2-X | Fast with good nucleophiles, unless there are branches at R- | |
| R’R”CH-X | Slow, is favored with good leaving groups and polar protic solvents. | Slow, is favored with good nucleophiles and aprotic solvents. |
| R’R”R»’C-X | Rapid with good protic solvents and protic groups | Highly unlikely |
| H2C=CH-CH2-X | Slow, favored with polar protic solvents | Fast, it is favored with good nucleophiles and aprotic solvents. |
| Ph-CH2-X |
Effect of the leaving group on the reaction
The leaving groups are weakly basic species that when the bond that keeps them attached to the carbon of the substrate is broken, they generate stable species. They can be anions or neutral molecules.
| Leaving group | Classification |
| -O-SO2-R (sulfonates) | Excellent |
| -I | Very good |
| -Br | Good |
| -Cl | Fair |
| -F | Poor |
| -OH, -NH2, -OR | Very poor |
The hydroxyl groups can be converted into good leaving groups, transforming them into the corresponding sulfonates.
Effect of the nucleophile on the reaction
Nucleophilicity is the ability of a group to act as a nucleophile in a substitution reaction. This ability is a function of the availability of electrons surrounding the nucleophile to attack the substrate.
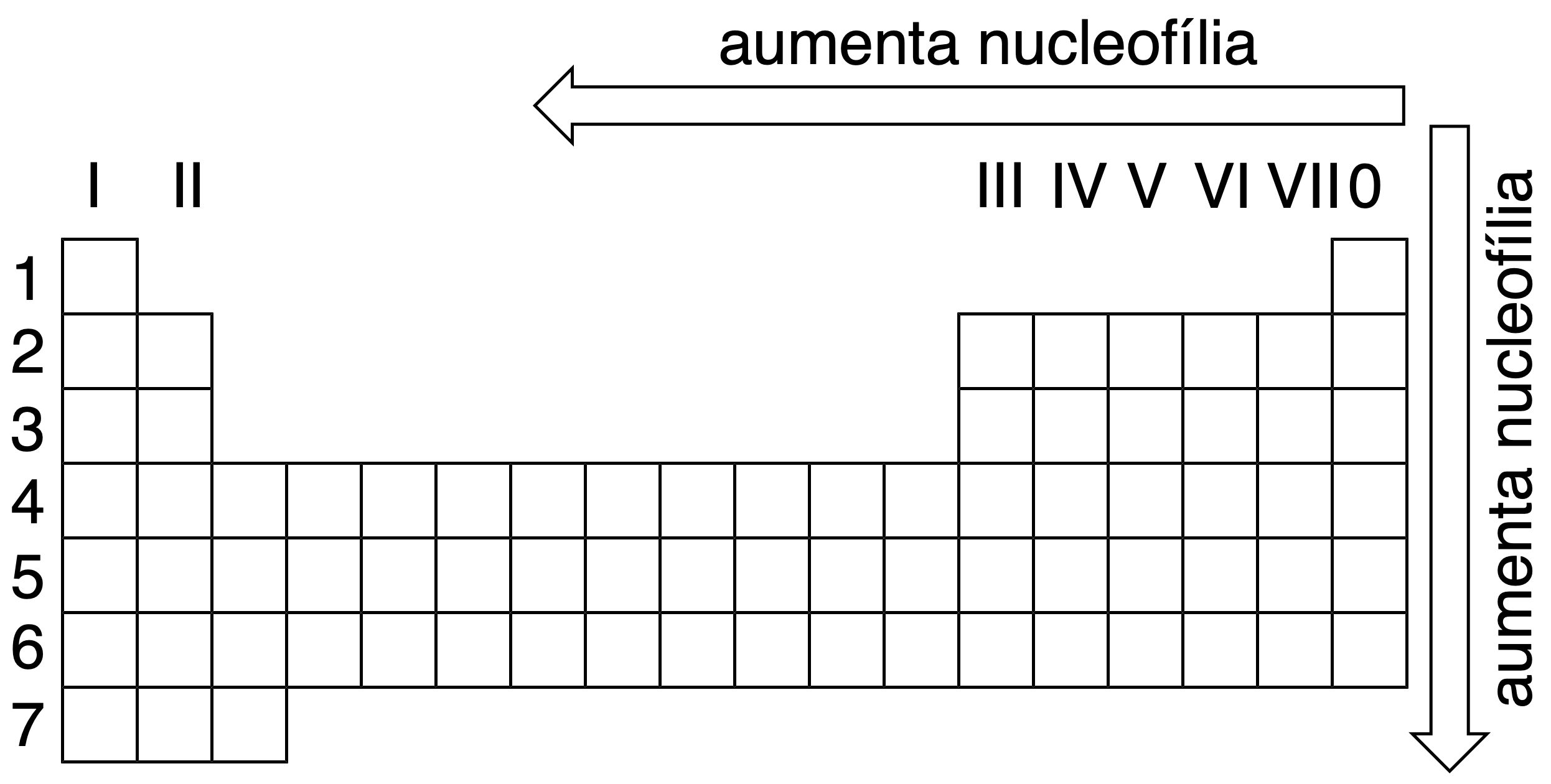
A comparison of the nucleophilic behavior of similar species, where a heteroatom is hydrogen bonded, in the same solvent, shows a variation along the periodic table as shown in the figure.
| Nucleophiles | Classification |
| HS–, RS–, PhS–, NH2–, HC≡C–, organometálicos | Very good |
| HO–, RO–, NH3, Br–, I–, CN–, N3– | Good |
| RCOO–, Cl– | Fair |
| H2O, ROH, F– | Poor |
Solvent effect on the reaction
Solvents that stabilize the intermediates (carbocations) or transition states in the SN1 and SN2, respectively, will favor the reaction.
SN1: it is favored with polar protic solvents (water, alcohols, carboxylic acids).
SN2: it is favored with moderately polar aprotic solvents (acetone, acetonitrile, DMF), provided that substrate and nucleophile are soluble.