Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is to obtain an azo dye, methyl orange, by the copulation reaction of a diazonium salt.
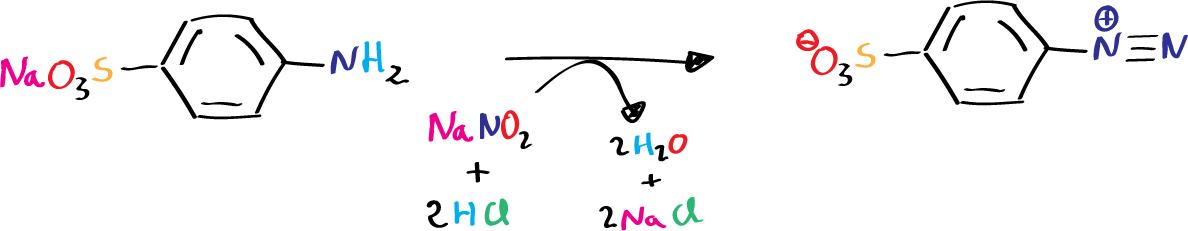
Background
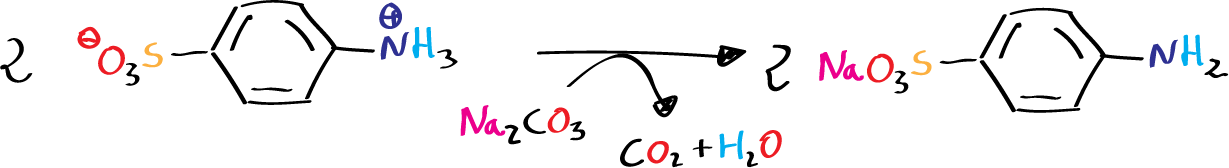
Each type of amine generates a different product when reacting with nitrous acid, HNO2. Unstable reagent formed in situ in the presence of the amine by the action of a mineral acid on sodium nitrite. When a primary aromatic amine, dissolved or suspended in a cold aqueous mineral acid is treated with sodium nitrite, a diazonium salt is formed.
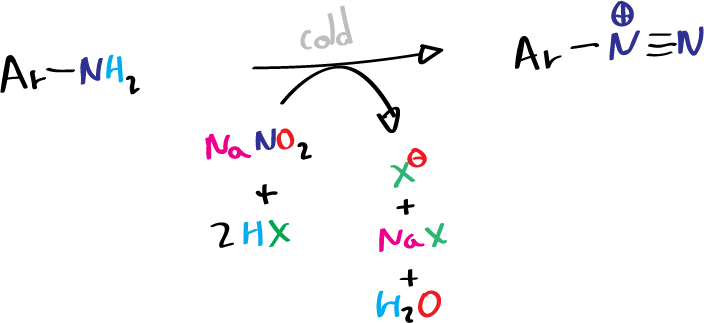
Since they decompose slowly, even at the temperature of an ice bath, their solutions are used immediately after preparation.
The large number of reactions that diazonium salts give can be grouped into two types.
- Substitution or replacement: in which the nitrogen is lost in the form of N2, leaving another group in its place in the ring.
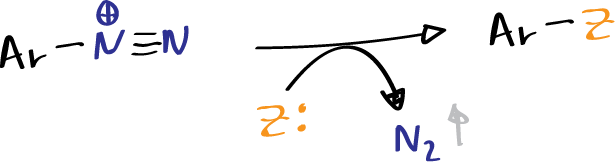
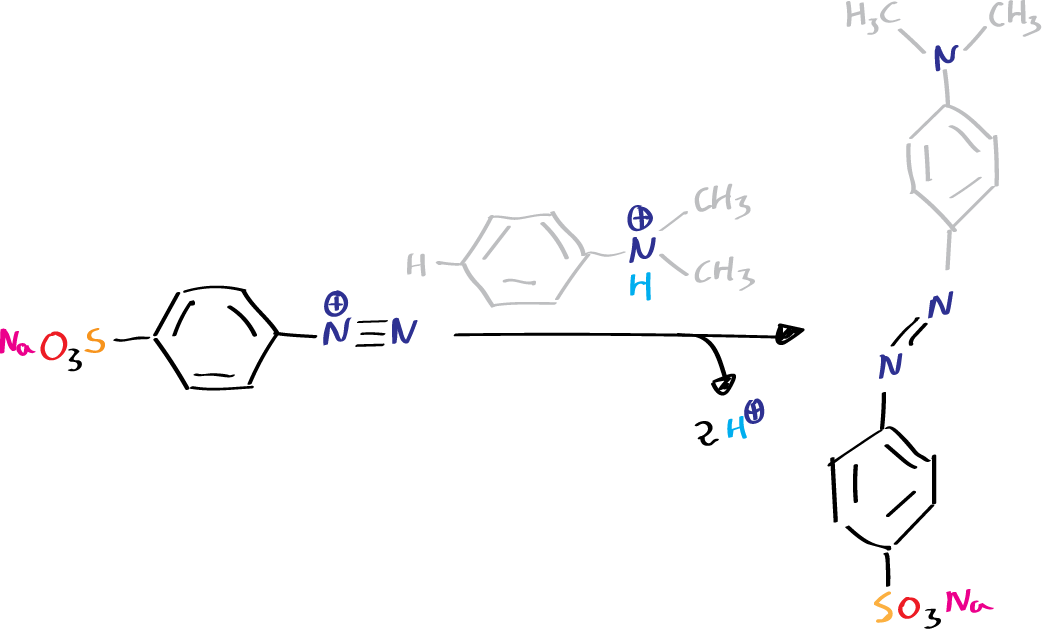
- Copulation: in which the nitrogen remains in the molecule. Copulation of diazonium salts with phenols and aromatic amines generates azo-compounds, which are key in the dye industry.
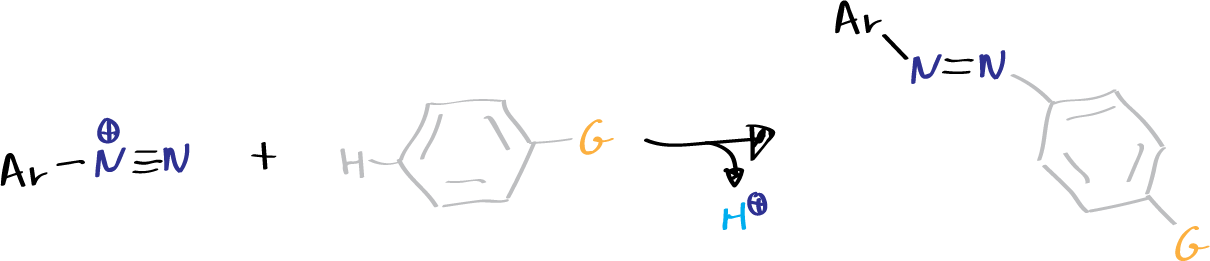
Obtaining a diazo dye consists of the following operations:
- Diazotization of an aromatic substance containing a primary amino group.
- Preparation of a solution of some amino-aromatic compound in a dilute acid or of a phenolic substance in a dilute alkali.
- Mixing of the above solutions with the consequent formation of the corresponding diazo dye, in a reaction called copulation. For this reaction to take place, the solution must be alkaline or slightly acidic.
In our case, to obtain methyl orange, we start by diazotizing sulfanilic acid, dissolve dimethylaniline in dilute HCl, and finally mix both solutions so that the copulation reaction can take place.
Experimental procedure
Prepare the following solutions independently:
- 5 g of sulfanilic acid (4-aminobenzenesulfonic acid) and 2 g of sodium carbonate in 100 ml of water.
- 2 g of sodium nitrite in 15 ml of water.
- 4 ml of concentrated HCl in 25 ml of water.
- 3 ml of dimethylaniline, 15 ml of water and 3 ml of concentrated HCl.
The solutions are cooled (with an external ice bath) and only when they are cold proceed as follows:
On the solution of sulfanilic acid and carbonate the solution of sodium nitrite is poured. The mixture is kept in an ice bath and the HCl solution is added slowly, with agitation. Once the addition is finished, the whole mixture is kept cold and the dimethylaniline solution is added slowly. It will be observed that the reaction acquires a reddish color.
Once the process is finished about 40 ml of NaOH at 10 % are added, with which the reaction mixture becomes orange, due to the formation of the sodium salt of the dye. Alkali is added until the solution becomes slightly alkaline (control the pH with indicator paper).
The reaction crude is transferred to a beaker of 500 ml and then 30 g of common salt are added and the content of the beaker is heated to 50-60 ºC. Once cooled to room temperature, the paste-like solid obtained is filtered under vacuum. A current of air is passed, in the Büchner, during 10 min. Once dry (it is recommended to use for this product a vacuum desiccator), to weigh the solid and to calculate the yield (estimated yield 70 %).
Take a small portion with the tip of a spatula and prepare an aqueous solution. Check what happens when an acid and base solution is added.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Methyl orange | 337.33 | >300 | - | - |
| N,N-Dimethylaniline | 121.18 | 1.8 | 192.5 | 0.958 |
| Na2CO3 | 105.99 | 851 | - | 2.532 |
| NaNO2 | 69.00 | 271 | 320 | 2.164 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Sulfanilic acid | 173.19 | >300 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| HCl |   |
| Methyl orange |  |
| N,N-Dimethylaniline |   |
| Na2CO3 |  |
| NaNO2 |    |
| NaOH |  |
| Sulfanilic acid |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Methyl orange | STZCRXQWRGQSJD-UHFFFAOYSA-M |
| N,N-Dimethylaniline | JLTDJTHDQAWBAV-UHFFFAOYSA-N |
| Na2CO3 | CDBYLPFSWZWCQE-UHFFFAOYSA-L |
| NaNO2 | LPXPTNMVRIOKMN-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Sulfanilic acid | HVBSAKJJOYLTQU-UHFFFAOYSA-N |
Video on the synthesis of methyl orange
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145
- Phenolphthalein and methyl orange
Charles A. Peters and Bryan C. Redmon
Journal of Chemical Education 1940 17 (11), 525
DOI: 10.1021/ed017p525