Written by J.A Dobado | Last Updated on May 2, 2024
What is one- and three-letter codes for amino acids?
Amino acids are the building blocks of proteins, and the specific sequence of amino acids determines the function and structure of the protein. To represent the 20 different amino acids, a system of codes has been established, with two commonly used formats: one-letter and three-letter codes.

The one-letter code uses a single uppercase letter to represent each amino acid. For example, Alanine is represented by the letter “A”, Arginine by “R”, and so on. This format is widely used in the field of bioinformatics and is convenient for representing amino acid sequences in a concise and standardized way. It is also commonly used in molecular biology experiments, such as in protein purification, where a single letter abbreviation can be used to label different fractions.
On the other hand, the three-letter code uses three uppercase letters to represent each amino acid. For example, Alanine is represented by “Ala”, Arginine by “Arg”, and so on. This format is used in many scientific publications, including research articles and textbooks, as well as in crystallography studies where it is used to label different amino acids in the protein structure.
While both one-letter and three-letter codes can be used to represent amino acids, each format has its own advantages and disadvantages. The one-letter code is more concise and easy to read, making it a popular choice for many applications. However, it can be difficult to interpret for individuals who are not familiar with the codes, and it may not always be appropriate for certain contexts, such as when the full name of the amino acid is needed.
On the other hand, the three-letter code is more descriptive and easier to interpret, making it a better choice for situations where clarity is important. However, it can be more cumbersome to use and may not be as standardized as the one-letter code, as different conventions have been established for different organisms and fields of study.
In conclusion, both one-letter and three-letter codes are widely used to represent amino acids, with each format having its own strengths and weaknesses. Researchers and scientists should be familiar with both formats in order to effectively communicate their work and collaborate with colleagues in the field.
Table for one-letter and three-letter amino acids codes
The following table shows the codes for amino acids, expressed in both one-letter and three-letter formats. The table lists all 20 amino acids, along with their corresponding codes in each format. The one-letter code is represented by a single uppercase letter, while the three-letter code uses three uppercase letters.
| Amino acid | Three letter code | One letter code |
| alanine | ala | A |
| arginine | arg | R |
| asparagine | asn | N |
| aspartic acid | asp | D |
| asparagine or aspartic acid | asx | B |
| cysteine | cys | C |
| glutamic acid | glu | E |
| glutamine | gln | Q |
| glutamine or glutamic acid | glx | Z |
| glycine | gly | G |
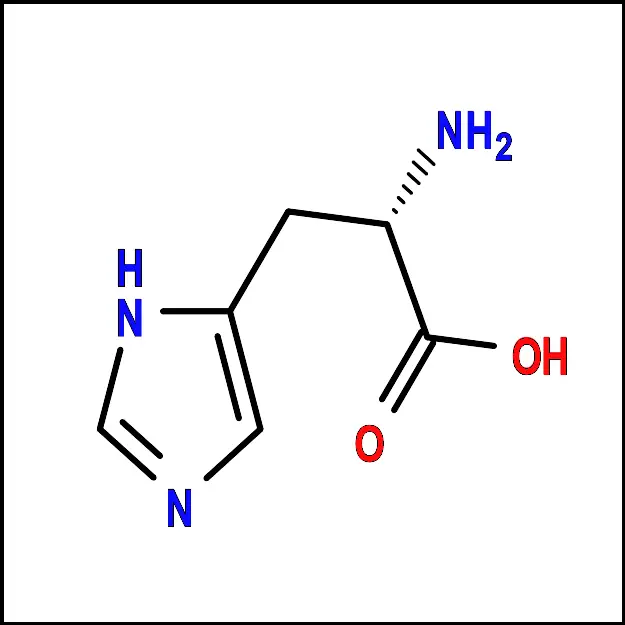
| histidine | his | H |
| isoleucine | ile | I |
| leucine | leu | L |
| lysine | lys | K |
| methionine | met | M |
| phenylalanine | phe | F |
| proline | pro | P |
| serine | ser | S |
| threonine | thr | T |
| tryptophan | trp | W |
| tyrosine | tyr | Y |
| valine | val | V |
For example, the first row of the table shows that the one-letter code for alanine is “A”, while the three-letter code is “Ala”, providing a quick and easy reference for researchers and scientists who need to work with amino acid sequences.
The codes for amino acids are important because they allow researchers to represent complex biological molecules in a concise and standardized way. This makes it easier to communicate research findings and collaborate with colleagues in the field. The one-letter and three-letter codes are both widely used, with each format having its own strengths and weaknesses. The one-letter code is more concise and easier to read, while the three-letter code is more descriptive and easier to interpret.
Example of a protein codification
Here is an example of the sequence of a short protein in both 3-letter and 1-letter code:
- Sequence: Met-Arg-Leu-Ile-Thr
- three-letter code: Met-Arg-Leu-Ile-Thr (or, in short: MRLIT)
- one-letter code: MRLIT
In this example, the protein consists of 5 amino acids: Methionine (Met), Arginine (Arg), Leucine (Leu), Isoleucine (Ile), and Threonine (Thr). The 3-letter code is used to spell out the full name of each amino acid, while the 1-letter code uses a single letter to represent each amino acid.
So, the sequence “Met-Arg-Leu-Ile-Thr” can also be represented as “MRLIT” using the 3-letter code, or as “MARIT” using the 1-letter code. Both representations convey the same information about the sequence of amino acids in the protein, but the 3-letter code provides more detail while the 1-letter code is more concise.
Twenty common amino acids
Amino acids serve as the fundamental units for constructing proteins within living organisms. While there exist more than 500 different types of amino acids in nature, the human genetic code specifically encodes only 20. Each amino acid has a unique structure, which determines its properties and role in protein synthesis.
Out of these 20, certain amino acids are considered “essential” as they cannot be produced by the body and must be acquired through dietary sources. Conversely, the remaining amino acids, known as “non-essential,” can be synthesized within the body.
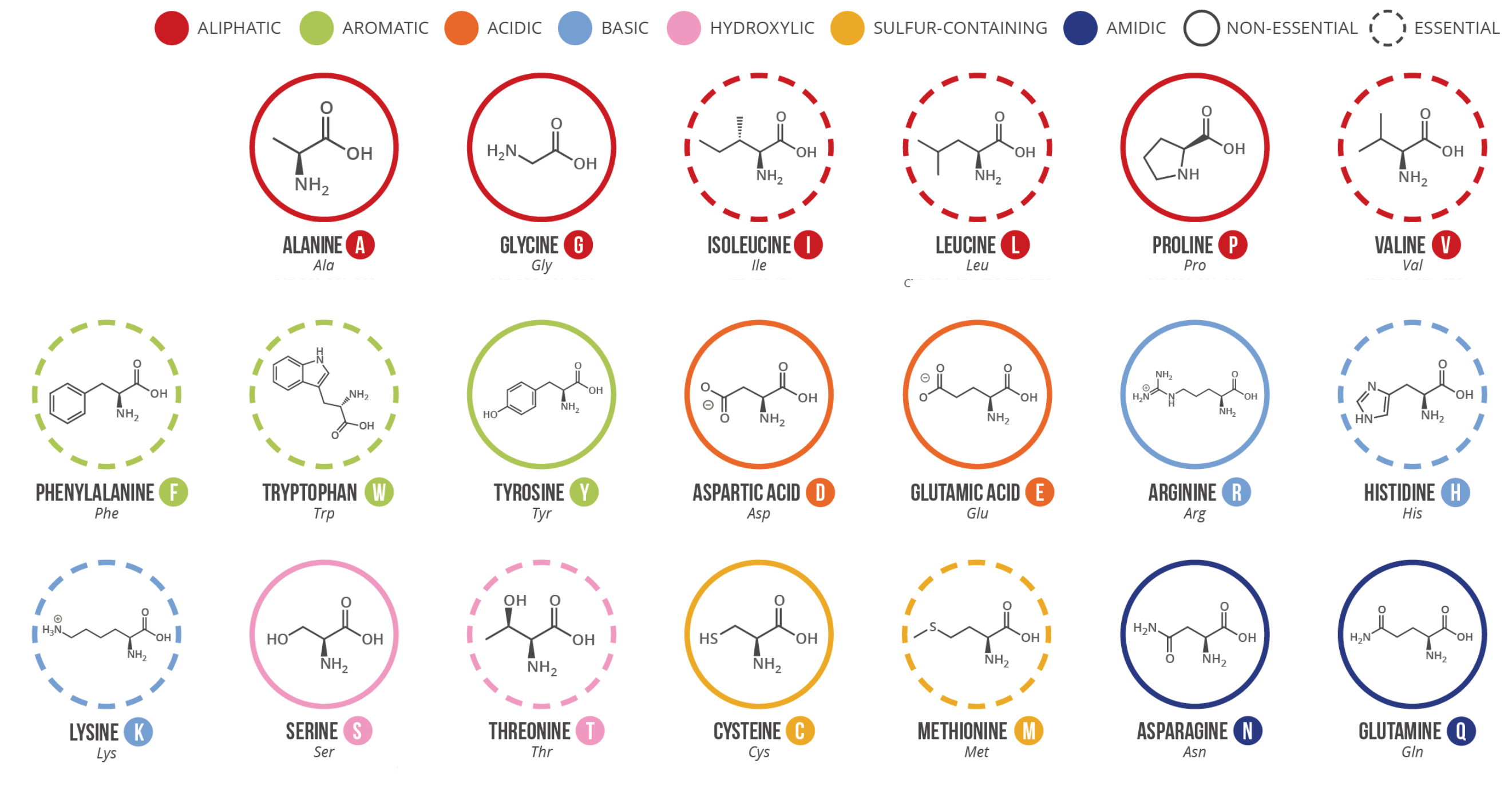
The amino acids displayed on the figure correspond solely to those directly encoded by the human genetic code. Although selenocysteine is sometimes referred to as the 21st amino acid, it is encoded in a unique way. Moreover, distinguishing between asparagine and aspartic acid, or glutamine and glutamic acid, can be challenging in certain instances. To address this issue, the codes asx (b) and gIX (L) are utilized for these amino acids, respectively.
Here is a brief overview of the 20 common amino acids:
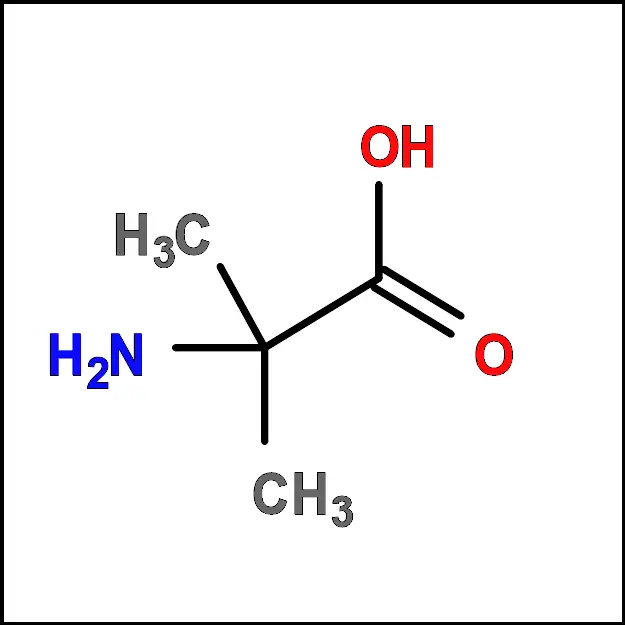
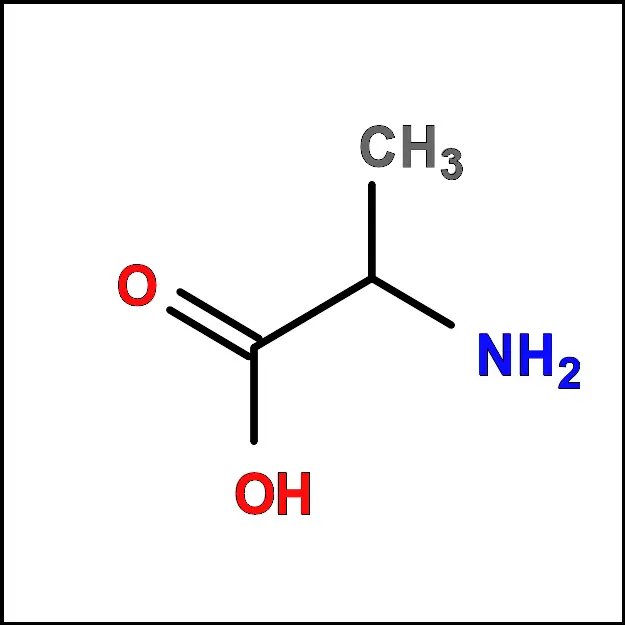
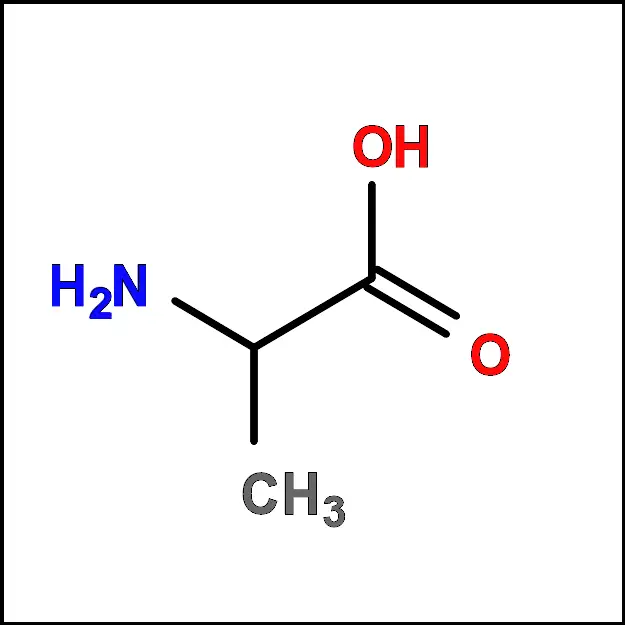
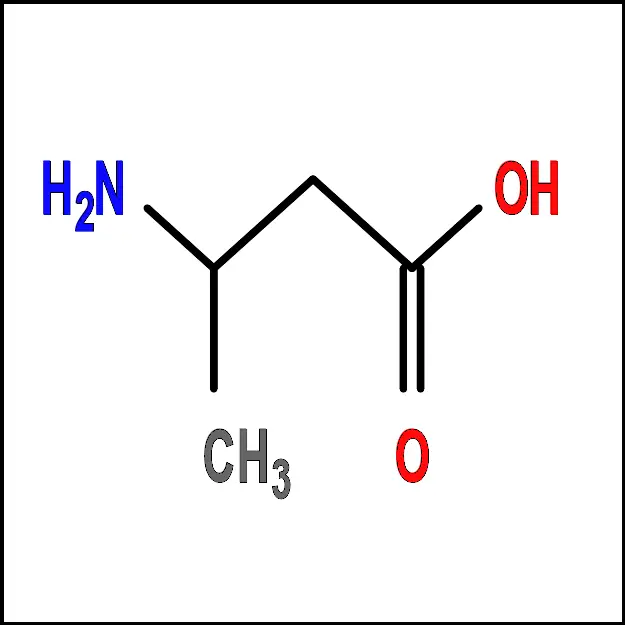
- Alanine (Ala) – A non-polar, aliphatic amino acid with a simple side chain consisting of a single methyl group (-CH3).

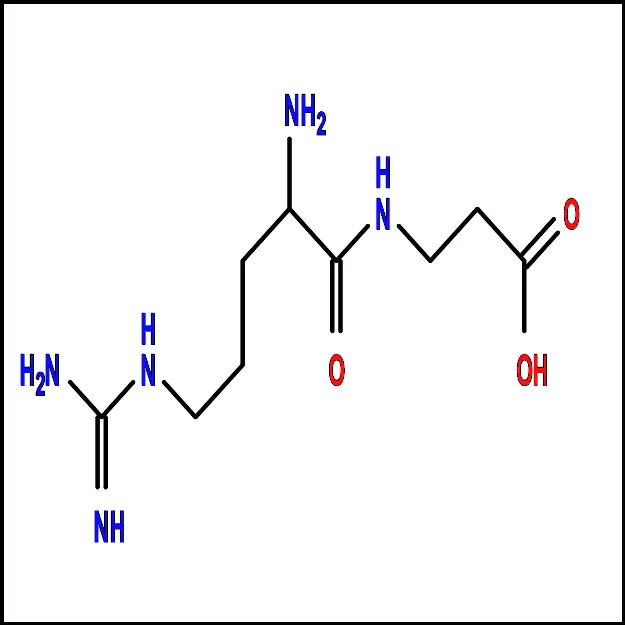
Alanine (Ala) - Arginine (Arg) – A polar amino acid with a positively charged side chain. It plays a key role in protein synthesis and is also involved in the urea cycle, which helps remove ammonia from the body.

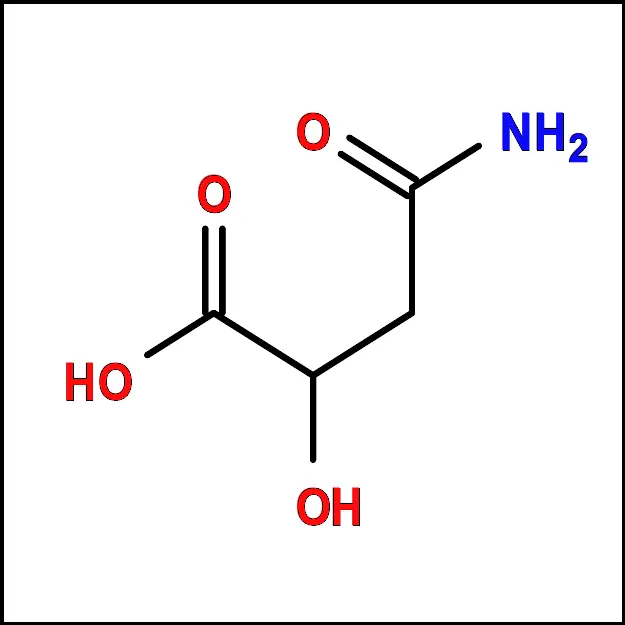
Arginine (Arg) - Asparagine (Asn) – A polar amino acid with a side chain that can form hydrogen bonds. It is important for protein folding and stability.

Asparagine (Asn) - Aspartic acid (Asp) – A polar amino acid with a negatively charged side chain. It is involved in many biological processes, including energy metabolism and neurotransmitter synthesis.

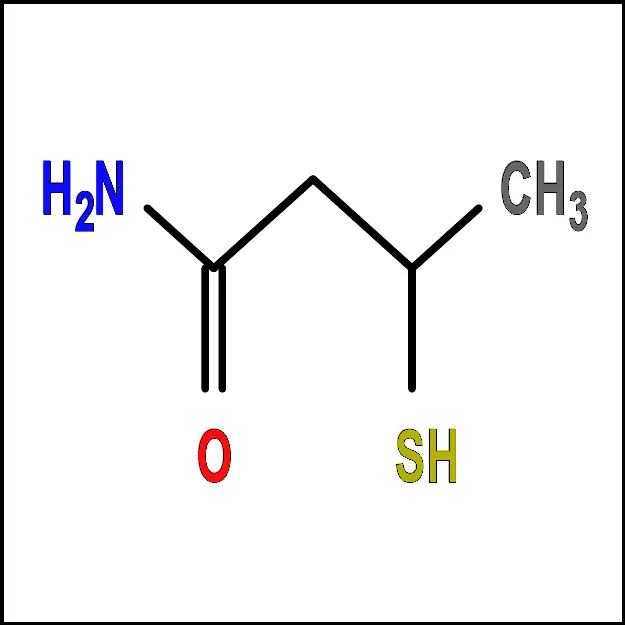
Aspartic Acid (Asp) - Cysteine (Cys) – A polar amino acid with a sulfur-containing side chain. It is important for protein structure and stability, as well as antioxidant defense.

Cysteine (Cys) - Glutamic acid (Glu) – A polar amino acid with a negatively charged side chain. It is involved in many biological processes, including energy metabolism and neurotransmitter synthesis.

Glutamic Acid (Glu) - Glutamine (Gln) – A polar amino acid with a side chain that can form hydrogen bonds. It is important for protein folding and stability.

Glutamine (Gln) - Glycine (Gly) – The smallest amino acid, with just a hydrogen atom as its side chain. It is important for protein structure and flexibility.

Glycine (Gly) - Histidine (His) – A polar amino acid with a positively charged side chain. It plays a key role in enzyme catalysis and is involved in acid-base balance in the body.

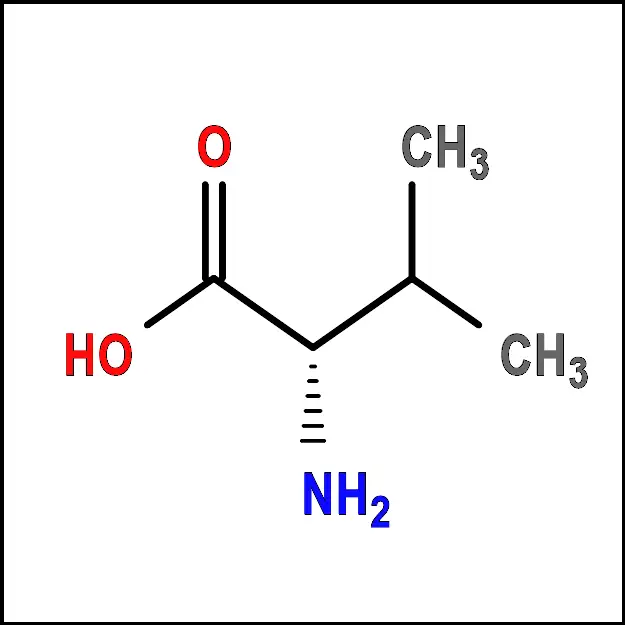
Histidine (His) - Isoleucine (Ile) – A non-polar amino acid with a branched side chain. It is important for protein synthesis and energy metabolism.

Isoleucine (Ile) - Leucine (Leu) – A non-polar amino acid with a branched side chain. It is important for protein synthesis and energy metabolism.

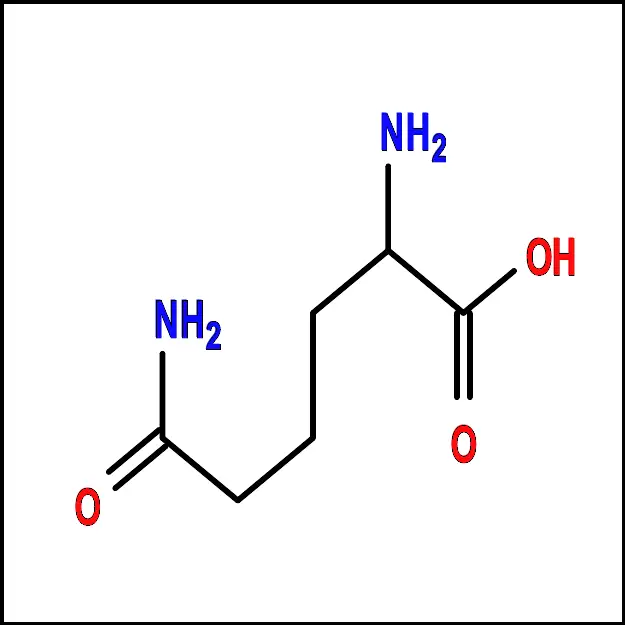
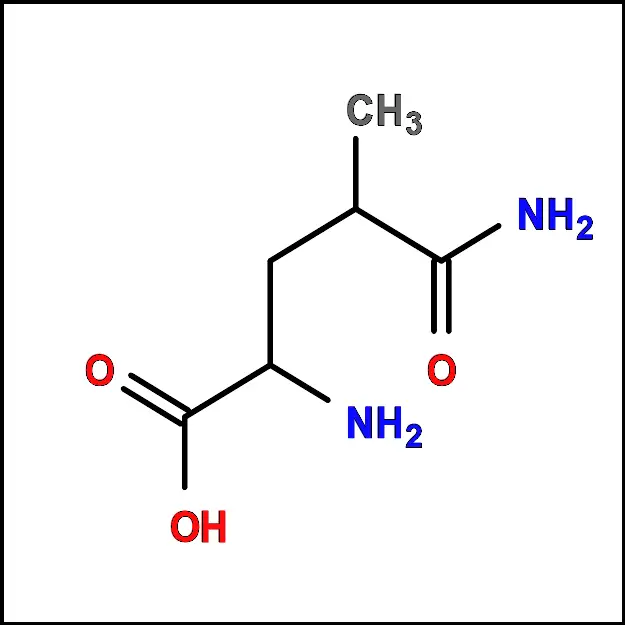
Leucine (Leu) - Lysine (Lys) – A polar amino acid with a positively charged side chain. It plays a key role in protein synthesis and is also involved in the urea cycle.

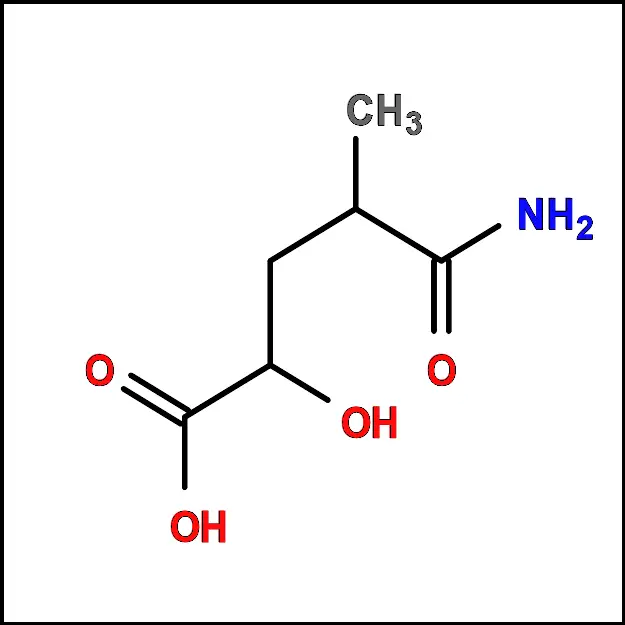
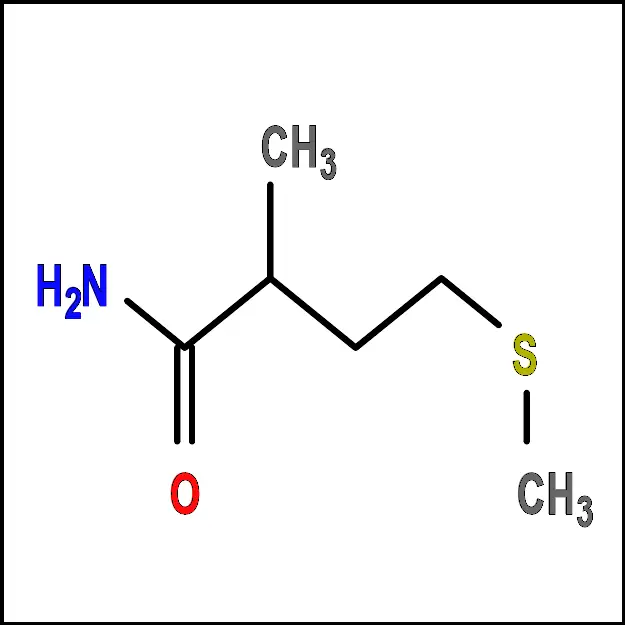
Lysine (Lys) - Methionine (Met) – A non-polar amino acid with a sulfur-containing side chain. It is important for protein synthesis and metabolism.

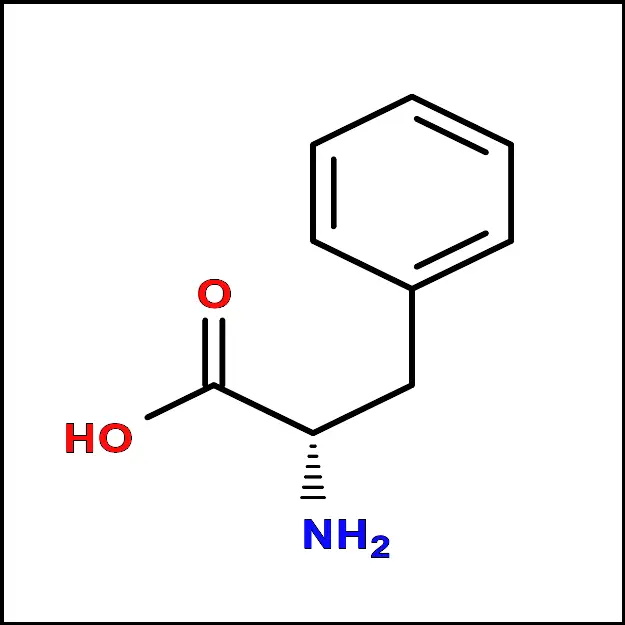
Methionine (Met) - Phenylalanine (Phe) – A non-polar amino acid with an aromatic side chain. It is important for protein synthesis and neurotransmitter synthesis.

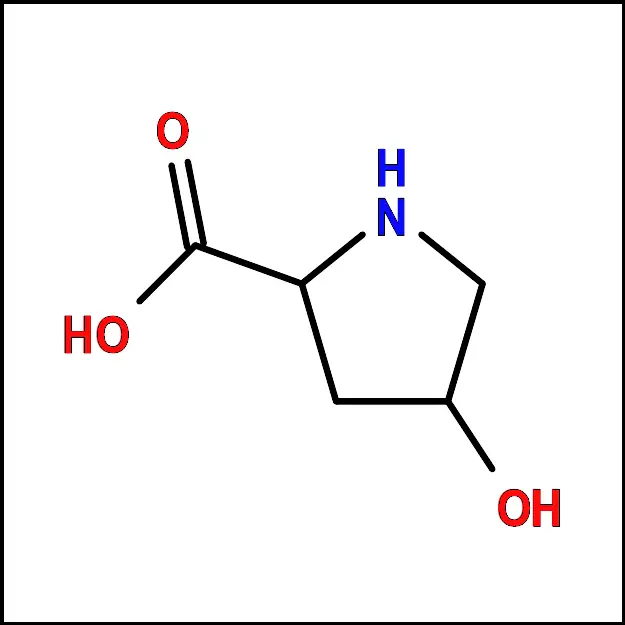
Phenylalanine (Phe) - Proline (Pro) – A non-polar amino acid with a cyclic side chain. It is important for protein structure and stability.

Proline (Pro) - Serine (Ser) – A polar amino acid with a side chain that can form hydrogen bonds. It is important for protein synthesis and metabolism.

Serine (Ser) - Threonine (Thr) – A polar amino acid with a side chain that can form hydrogen bonds. It is important for protein synthesis and metabolism.

Threonine (Thr) - Tryptophan (Trp) – A non-polar amino acid with an aromatic side chain. It is important for protein synthesis and neurotransmitter synthesis.

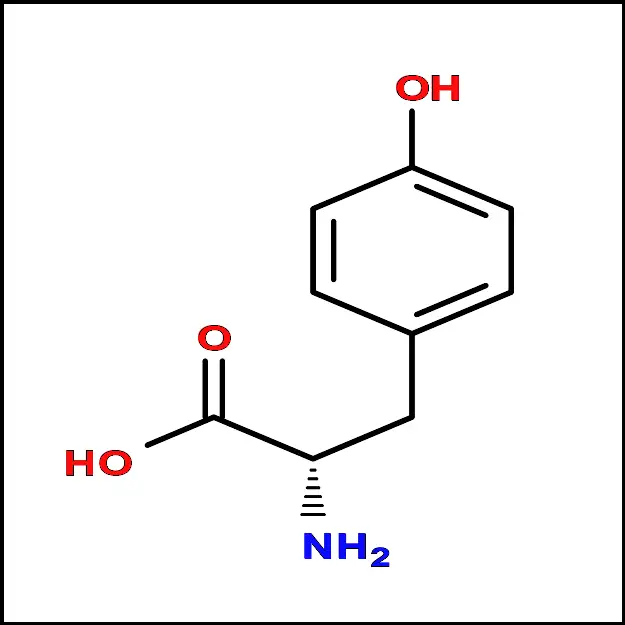
Tryptophan (Trp) - Tyrosine (Tyr) – A polar amino acid with an aromatic side chain. It is important for protein synthesis and neurotransmitter synthesis.

Tyrosine (Tyr) - Valine (Val) – A non-polar amino acid with a branched side chain. It is important for protein synthesis and energy metabolism.

Valine (Val)