Written by J.A Dobado | Last Updated on May 2, 2024
What is Zerewitinoff determination?
The Zerewitinoff determination is a method to quantitatively determine the active hydrogens in a chemical substance by adding methylmagnesium iodide in pentyl ether to the solution of substrate and measuring the volume of gaseous methane evolved. The reaction was first reported by Chugaev and subsequently extended by Zerewitinoff. The method is also known as the Zerewitinoff test, Zerevitinov determination, Zerevitinov test, Zerevitinov hydrolysis, Zerevitinov reaction, Tschugaeff-Zerewitinoff determination, Zerewitinoff method, Zerevitinov method, and Tschugaeff-Zerewitinoff determination.
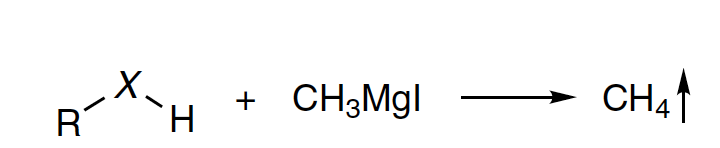
Chugaev did not design an apparatus for measuring active hydrogens using methylmagnesium iodide, and it was Hibbert and subsequently Zerewitinoff who reported devices to mix the Grignard reagent and substrate in the same glass container and measure the volume of methane. Zerewitinoff extended the method to determine active hydrogens of compounds with hydroxyl, amino, imino, and amido groups.
Most active hydrogen determinations have been achieved using methylmagnesium iodide due to its easy preparation. Molecules that react with methylmagnesium iodide fall into two groups: those bearing active hydrogen(s) and those that don’t produce methane. Group a molecules contain functional groups of hydroxyl (alcohols and phenols), sulfhydryl (thiols and thiophenols), carboxyl, amino, amido, etc. Among these molecules, alcohol, thiols, secondary amines (including pyrroles), and monosubstituted amides liberate 1 mol methane, whereas water, primary amines, and amides produce 2 mol methane per mole of substrate. The Grignard reagent usually adds or couples with group b molecules, which include aldehydes, ketones, nitriles, isonitriles, esters, and acyl halides (all involving addition) as well as alkyl halides, tosylate, and triflates (SN2 substitution). Among group b molecules, esters and acyl halides react with 2 equivalents of Grignard reagent, whereas acids need 3 equivalents of methylmagnesium iodide, while the remaining molecules react with a stoichiometric amount of Grignard reagent. However, certain molecules can be classified into both groups due to keto-enol tautomerization. For example, β-diketones, including acetoacetone, benzoylacetone, and ethyl acetoacetate, ethyl malonate, all show one active hydrogen at 100 ºC, but liberate much less methane at 20 ºC. Likewise, resorcinol bearing two hydroxyl groups is tested to have only one active hydrogen in pyridine, though it is determined normally in anisole.
Typically, the Zerewitinoff determination takes between 5 and 30 minutes to complete, and its results are usually accurate and reproducible within a range of ±3 to 5%.
Applications
Before modern instrumental analysis was introduced, Zerewitinoff determination was extensively used in the initial stages of structural analysis for organic compounds. Furthermore, this reaction is highly beneficial for assessing the concentration of newly prepared Grignard reagent and the stability of Grignard reagents that are kept in storage for extended periods of time.
References
- Chugaev, T., Ber., 1902, 35, 3912
- Zerevitinov, Th., Ber., 1908, 41, 2233
- Zerevitinov, Th., Ber., 1910, 42, 4802
- Zerevitinov, Th., Ber., 1911, 43, 3590
- Zerevitinov, T., Z. Anal. Chem., 1914, 52, 729
- Zerevitinov, Th., Ber., 1914, 47, 2417
- Zerevitinov, Th., Z. Anal. Chem., 1926, 68, 321