Written by J.A Dobado | Last Updated on April 22, 2024
Oxidation-reduction concept
Initially, these processes were understood as follows:
Oxidation: addition of oxygen to a substance.
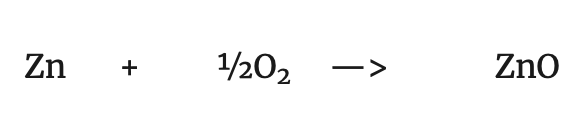
Reduction: Loss of oxygen from a compound, resulting in a reduction in mass:
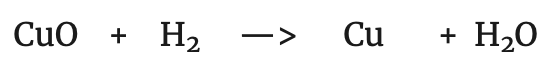
If the reaction of zinc in the first process is compared with the following:
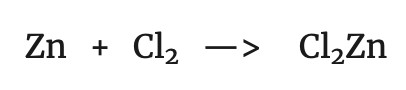
It can be seen that the following transformation has taken place in both:
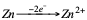
That is to say that Zn has lost two electrons. On the other hand, if we compare the reaction of copper with the following:
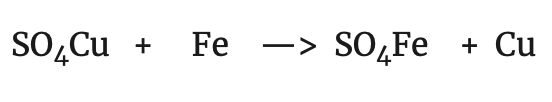
we observe that in both cases copper has undergone the same change:
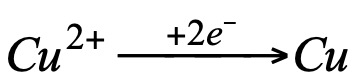
i.e. copper has gained two electrons. In view of these observations, a new concept of oxidation and reduction was established:
- Oxidation: Process in which electrons are lost.
- Reduction: Process in which electrons are gained.
Since electrons do not exist free, for a substance to gain electrons there must be another substance that loses them, i.e., both redox processes are linked.
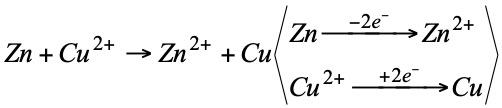
Zn is oxidized (reductant).
Cu2+ is reduced (oxidizer).
- Oxidizer: A substance that is easily reduced, since it readily accepts electrons from another, which, when it loses them, oxidizes.
- Reductant: A substance that oxidizes easily, since the electrons it loses are given up to another substance, which, upon taking them up, is reduced.
An oxidation reaction cannot take place without a reduction reaction or vice versa. These oxidation-reduction systems are called “redox systems”. To easily identify whether a reaction is redox or not, we must familiarize ourselves with the concept of oxidation number.
In a redox system we can distinguish two half-reactions:
a) Reduction of the oxidant (X):
b) Oxidation of the reductant (M):
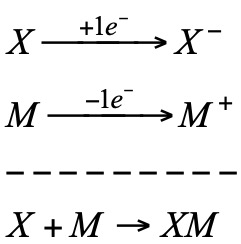
In the redox process there is an electronic transfer from the reductant to the oxidant. This transfer can take place without the need for the reactants to be in direct contact, a suitable electrical connection between them being sufficient.
In many cases, the reaction is almost completely displaced in one of the directions. If a substance behaves as a strong oxidizer (1) its reduced form (1) will be a weak reductant; conversely a strong reductant (2) will have an oxidized form (2) which will be a weak oxidant:
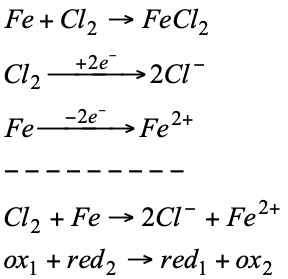
Balancing a redox reaction by the ion-electron method
First, the redox equation is separated into two partial equations:
- The formula of the oxidant (ion or molecule) is written and then its reduction product (ionic or molecular).
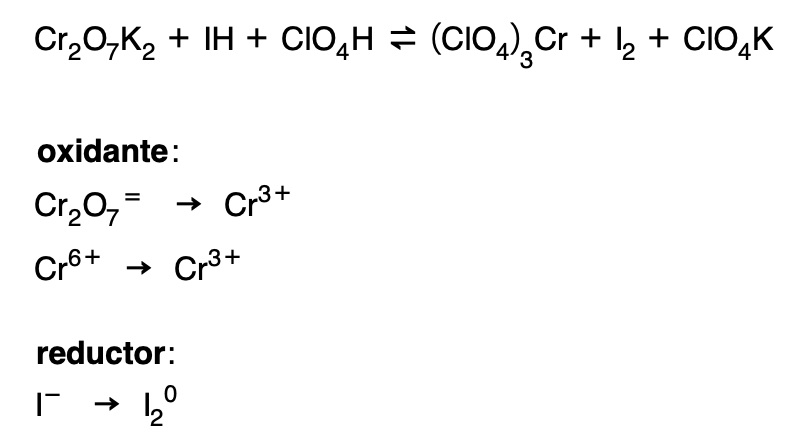
2. We introduce the minimum coefficients necessary to balance atoms of the characteristic element:

If the reduced form has less oxygen, it is introduced in the form of water in the second member:

Adding the necessary H+ in the first member:
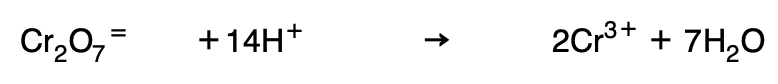
4. Balance the reaction electrically (enough electrons are introduced in the first member), and once balanced, the arrow is replaced by the equal sign.
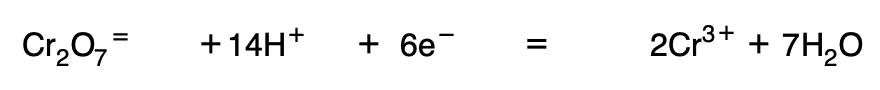
5. The same process is repeated but this time for the reducer, but now H2O would be added if necessary in the first term, and in the second term the H+ and electrons would appear:
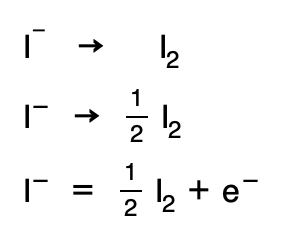
6. They are multiplied by the minimum coefficients necessary for the number of electrons to equalize:
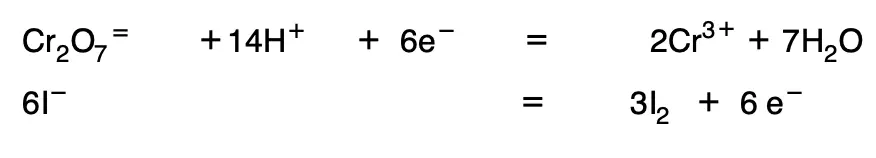
The two equations are then added together and the water and H+.
- If in the final equation there are still H+ in the second member an equal number of HO– are added to both members so that the H+ are converted into H2O. In such a case the reaction will take place in alkaline medium, so that the oxidation of the reductant will take place by HO–.
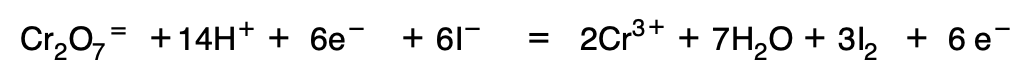
8. Ions that do not undergo a change in their oxidation number are introduced:
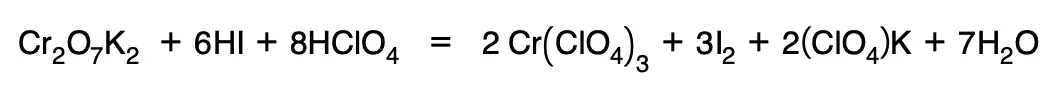
Redox volumetries. Equivalent of an oxidant or a reductant.
Equivalent weight, equivalent mass or equivalent of an oxidant or a reductant is the quantity of a substance that gains or gives up an electron. It is calculated with the following expression:
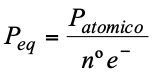
For example:
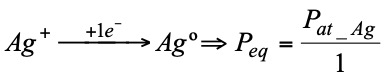
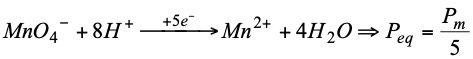
It should be remembered that:
- The redox equivalent is only applicable when the substance is involved in processes of this type.
- The conditions under which the reactions take place influence the nature of the final products and therefore the number of electrons transferred. Therefore, to determine the redox equivalent it is necessary to know not only the starting product but also the product obtained:
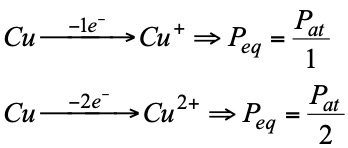
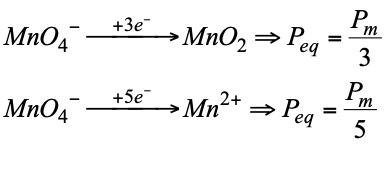
The normality of oxidizing or reducing substances: it is obtained in the same way as for the others, but using the concept of equivalent that we have just seen.
For example:
Exercise: Calculate the amount of potassium dichromate to be weighed to prepare 1 liter of 0.1 N solution when this substance is to be reduced to Cr3+ ion ( Cr 52; O 16; K 39).
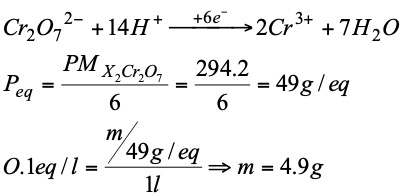
The redox volumetry consists of taking advantage of these reactions to determine the concentration of one of them.
Example: suppose we have a sample of 25 cm3 of a Fe2+ solution and we want to know the Fe2+ content of this solution. The 25 cm3 of the Fe2+ solution to be titrated are placed in the Erlenmeyer flask with a few drops of H2SO4 and KMnO4 of known concentration (0.1027 N) (Mn 55; O 16; K 39) is added dropwise from the burette. The following reaction will take place:
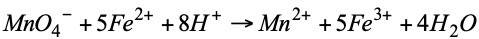
In this case no indicator will be needed since KMnO4 is violet and Mn2+ is almost colorless, on the other hand Fe2+ is somewhat greenish and Fe3+ is yellowish. As KMnO4 is added, the violet color will disappear as it changes to Mn2+. When all the Fe2+ of the Erlenmeyer is exhausted and we add one more drop of KMnO4, the violet color will remain in the solution, at this moment the volume of KMnO4 spent (21.6 cm3) is noted down.
In the redox reaction has occurred:
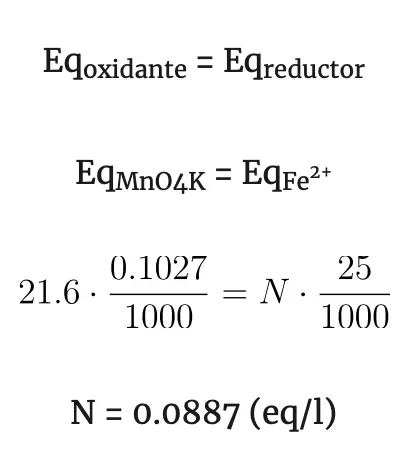
Electrochemical cells. Electromotive force. Electrode potentials
The process by which electric current is produced from chemical reactions. Assume the reaction:
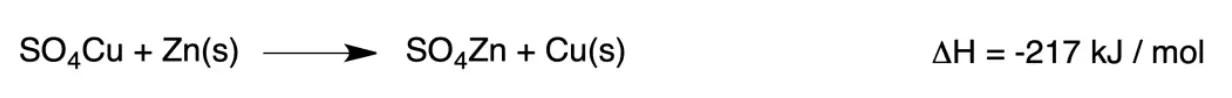
If the reaction were carried out directly in the same vessel it would be observed that the solution, originally blue (due to the copper sulfate), loses color as its temperature rises and metallic copper is deposited at the bottom. (A reddish-brown, spongy deposit forms on the zinc).
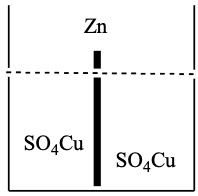
The semi-reactions that take place are:
There is a transfer of electrons from Zn to Cu. If this transfer is achieved not directly but through an external circuit, we will have obtained an electric current. The device thus constructed is the Daniell cell or galvanic electric cell, capable of setting charges in motion through a circuit, taking advantage of the energy released in a chemical reaction.
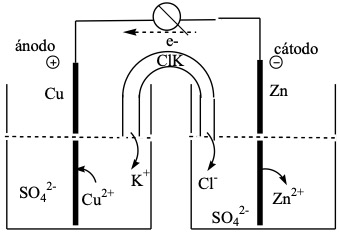
In order for the electrons to circulate through the external circuit, the reactants are separated into two parts called half-stack or half-elements. In this cell, both half-elements are connected by means of a U-shaped tube filled with an ionic compound (KCl) called salt bridge, whose mission is to cancel the excess of positive and negative charges that originate in the half-elements, allowing the K+ and Cl– ions to pass through the porous membrane that closes it.
Pila de Daniell:
To build a battery it is necessary to have two semi-elements in which there is a different capacity to oxidize or reduce, so that the electrons circulate from one to the other semi-element. In this reaction an energy has been given off which will depend on the nature of the reacting products and the initial and final states of the system.
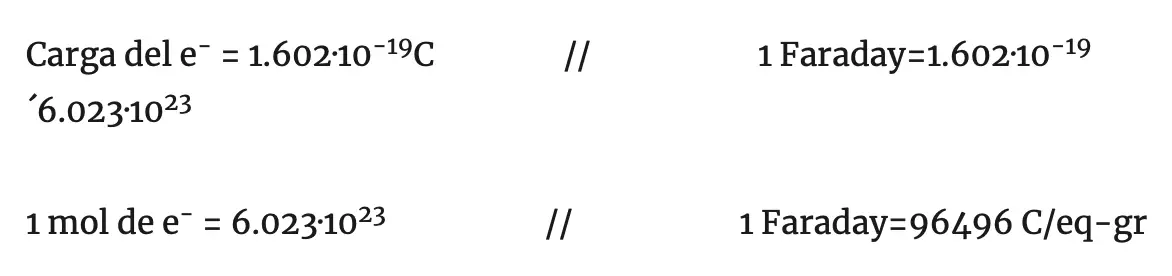
The charge of a mole of electrons is called Faraday.
(Volt = Joule / Coulomb)
Each generator or battery develops a certain amount of energy for each Coulomb it circulates, such energy per unit charge is called electromotive force of the generator (emf).
As we already know, a battery is made up of two half-elements or half-batteries called electrodes.
For example, the Daniell battery consists of a copper electrode (metallic copper in a solution of its ions) and a Zn electrode (metallic zinc in a solution of its ions). According to the nature of these electrodes the battery will present a certain value of its emf. It depends on the temperature, concentration and chemical nature of the substance.
To determine the emf of the different batteries, a certain electrode is chosen as reference electrode and these emf are referred to it. The electrode chosen as the reference electrode is the hydrogen electrode, which is called the standard electrode.
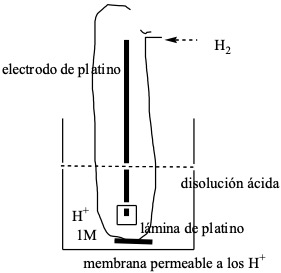
This electrode is achieved by using a platinum foil, on which pure hydrogen is bubbled at P = 1 atm, and which is introduced into an acidic solution at which the proton concentration is [H+] = 1 M.
The reaction that takes place at this electrode is:
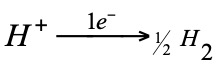
When it is desired to determine the normal potential of any electrode [1M solution, 1 atm pressure (gas) and pure solids (more stable forms at 15ºC)], the battery is assembled with the corresponding semielement and the hydrogen normal (zero volts potential).
For the notation of a battery, each element is written separated by a line, from the ions of the solution and two dashes, separating the two solutions, placing on the left the electrode where the oxidation of the reductant (anode) takes place and on the right the electrode where the reduction of the oxidant (cathode) takes place. According to this convention, the notation of the Daniell cell is:
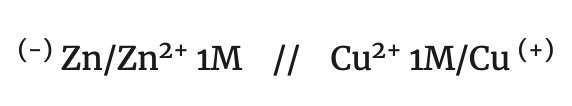
anode/anode electrolyte // cathode electrolyte/cathode
This battery in standard conditions gives a emf of 1.10 v.
Depending on whether the normal hydrogen electrode acts as the negative or positive pole of a battery, the reaction that will take place will be as follows.
Negative pole (anode) oxidation:
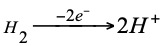
Positive pole (cathode) reduction:
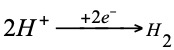
To measure the relative potential of any isolated electrode, two data must be collected:
- the emf of the stack formed by this electrode and the normal hydrogen electrode.
- Whether the electrode we are measuring is the positive or negative pole of the battery.
From the first data the numerical value and from the second the sign of the electrode potential is deduced.
For example: If we want to determine the potentials of the normal zinc and copper electrodes, we have to form the respective stacks with the normal hydrogen electrode.
Measuring the emf of these batteries we obtain a value of 0.76 V for the first one and 0.34 V for the second one. But in the first case, the zinc electrode is the negative pole and, in the second case, the copper electrode is the positive pole.
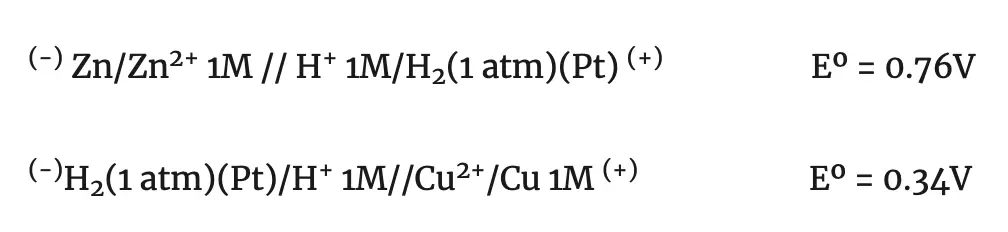
Therefore, the normal reduction potentials of the zinc and copper electrodes are:
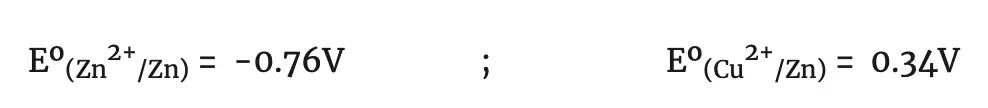
Daniell cell 1.10V Eº(Cu2+/Cu) = 1.10-0.76 = 0.34 V
The redox reactions taking place in both stacks are:
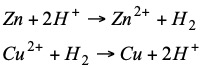
Any electrode immersed in a 1M solution of its ions is called normal electrode and its potential (against the normal hydrogen electrode) with its corresponding sign is called normal or standard potential and is tabulated. The normal reduction potentials are tabulated and when their sign is negative it means that the indicated reaction is not spontaneous.
The higher the reduction potential, the greater the tendency of the electrode to reduce, i.e. the greater its oxidizing power.
Knowing the normal reduction potentials of the semielements, the emf of a battery, formed by any pair of them, can be calculated and the polarity of the same can be predicted. To do this it is sufficient to write the electrode reactions and their respective potentials, with the appropriate signs.
The negative pole will be the electrode with the lowest potential, that is, the most negative, which will tend to yield electrons, while the positive pole will be the one with the highest potential, which will tend to capture electrons.
The emf will be the sum of both potentials and the overall reaction of the stack will be the sum of the corresponding reactions.
Redox processes in electrolytic cells
In the section on electrochemical cells it has been described how electric current can be produced by taking advantage of chemical reactions. This section details the reverse electrolytic process, i.e. how a non-spontaneous chemical reaction can be produced by means of an electric current.
Electric current is the movement of electric charges. In an electrical circuit (metal wire) electrons do not disturb the conducting wires (although some heating does occur). This is due to the types of bonding that are present in metals (metallic bonding which gives rise to metallic or electronic conduction).
However, the passage of electricity through solutions of some salts (or molten salts) causes chemical changes in the dissolved electrolyte, the electrodes, or both, as chemical reactions occur.
For example, solid NaCl does not conduct electric current because the Na+ and Cl– ions are fixed in the crystal lattice and cannot move. However, molten solid NaCl does conduct electric current because the ions are now.
In electrolysis, the conduction of electric current is due to the migration of ions (rather than the movement of electrons), and is referred to as ionic or electrolytic conduction.
NaCl, if we increase the temperature above 800 ºC, which is the melting point of NaCl, and introduce two inert graphite electrodes connected to the poles of a direct current generator, if the voltage is sufficient.
In this case, Na+ ions will take electrons from the cathode and Na will be deposited as metallic sodium. At the same time, the negative Cl– ions will give up electrons to the anode and Cl2 will be released as a gas. The reactions that take place are summarised below:
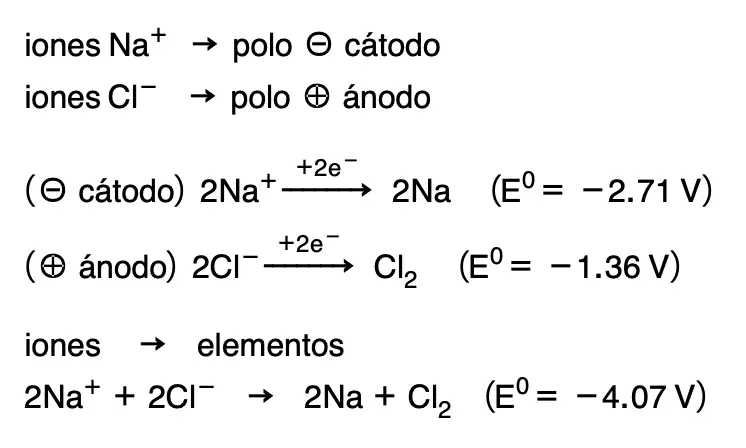
The theoretical minimum potential of this reaction would be -4.07 V, and the inverse reaction to the one that occurs in nature would take place, that is to say, from ions we would obtain with electric current the elements Na and Cl2. In both galvanic cells and electrolytic tanks, the anode is the electrode where oxidation occurs, and the cathode is where reduction occurs.
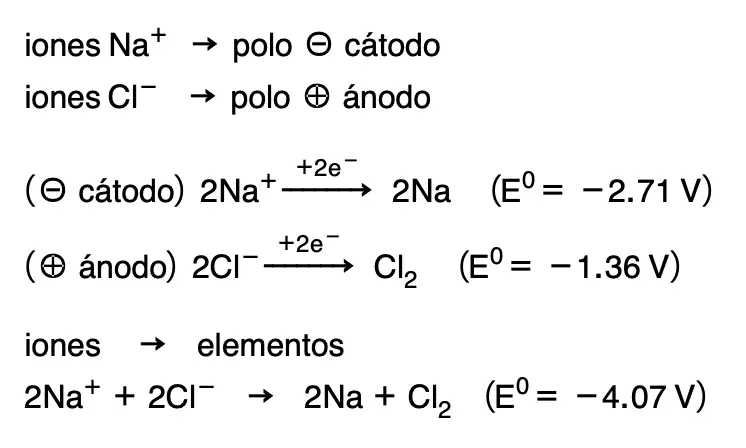
The reactions that take place in electrolytic cells are redox processes. For example: electrolysis of copper(II) chloride. If two inert Pt electrodes are introduced and connected to the terminals of a current generator and the following is observed:
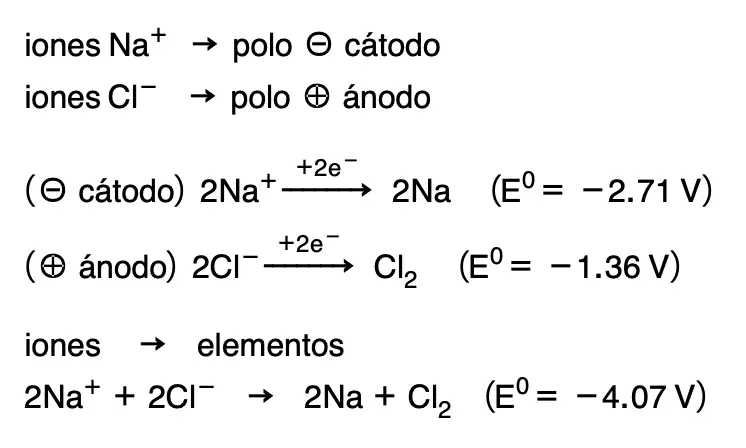
Each electrode is charged with the sign of the terminal to which it is attached. The ⊕ electrode is the anode and the ⊖ electrode is the cathode.
The ions in the solution by electrical forces, are directed towards the electrodes: the anions ⊖ go to the anode ⊕ and the cations ⊕ to the cathode ⊖.
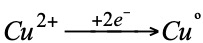
anode ⊕: electron acceptor (reduction of the oxidizer)
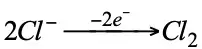
cathode ⊖: electron donor (reductant oxidation)
The greater the electromotive force (emf) of the generator, the greater the extent of the process.
The reactions that take place in the electrolytic tank as a consequence of the supplied electrical energy consist of the decomposition of the substances and the process is called electrolysis.
However, the electrolysis process is not spontaneous, i.e., the decomposition would not occur if no electrical energy had been supplied. FARADAY is defined as an amount of charge equal to 96496 C capable of decomposing an equivalent of any electrolyte.
Nerst equation: relation between the potential and the equilibrium constant of a redox reaction
If the reactants or products of the corresponding redox reaction are at different conditions (temperature, pressure, concentration, etc.) the emf will be different.
We are going to see the relationship between the potential of a redox reaction and the concentration of the species involved, that is, between the equilibrium constant.
As a consequence of a spontaneous reaction we obtain a current in a redox reaction. This relationship led Thomson and Helmholtz to think that the origin of the electrical energy came from the chemical energy of the process (heat of reaction).
As in both cases the reaction is carried out at constant pressure (p atmospheric):
Electrical energy = heat of reaction
n·F·E = Qp
de donde E = Qp/n·F regla Thomson-Helmholtz (1851) [1]
Qp is the heat of reaction expressed in joules (1 cal =4.1840 J).
E is the potential difference in volts.
F is Faraday 96496 C/eq-gr
n is the number of formed equivalents
This electrical energy can be quantified by means of electrical work:
W = I·E·t = (Q/t)·E·t
I = intensity; E potential; t time
W = Q·E
W= z·E·F = Qp = -ΔH
Q = z·F; z = nº electrons; F charge of a mole 96496 C
E =-ΔH/z·F
This is the ratio of the potential of a reaction to its heat of reaction. It is an approximate expression, since the electrical energy of a spontaneous reaction is related to ΔG rather than to ΔH.
E = -ΔG/z·F
On the other hand we know that:
-ΔG = RTln(K/J)
K = cte equilibrium; J = cte in which reactants and products appear at the current time and not at equilibrium.
E = (R·T/z·F)ln (K/J)
E = (R·T/z·F)ln K-(R·T/z·F)ln K F = 96496
If the temperature remains constant, we will have that:
E = (R·T·2.303/z·F)log K-(R·T·2.303/z·F)log K R·T·2.3/F = 0.06
Factors affecting the potential of a redox system
If we consider the following reaction:
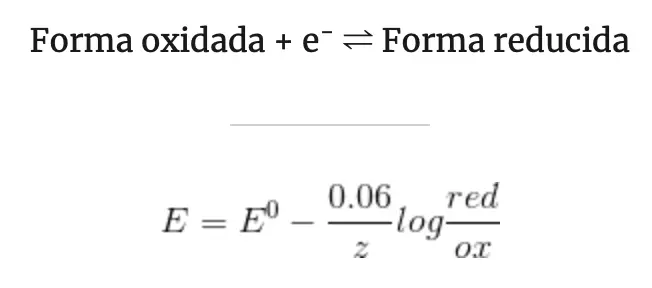
A variation in concentrations will produce a variation in potential. Therefore, factors that affect the concentration will affect the potential. For example:
pH
1) In those systems in which the oxygen in the reduction process varies:
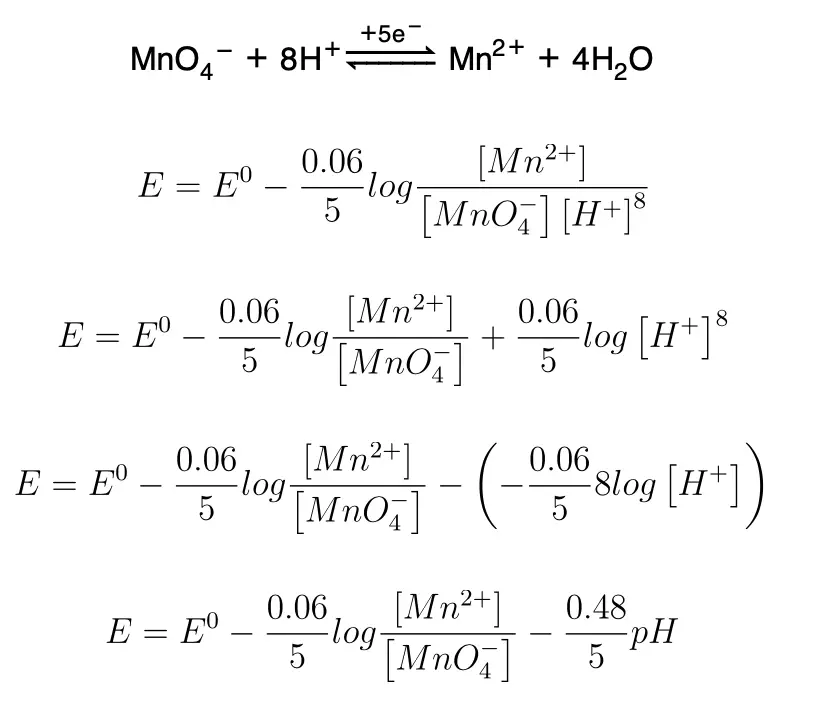
The following graph shows that the potential E will be maximum when pH is zero.
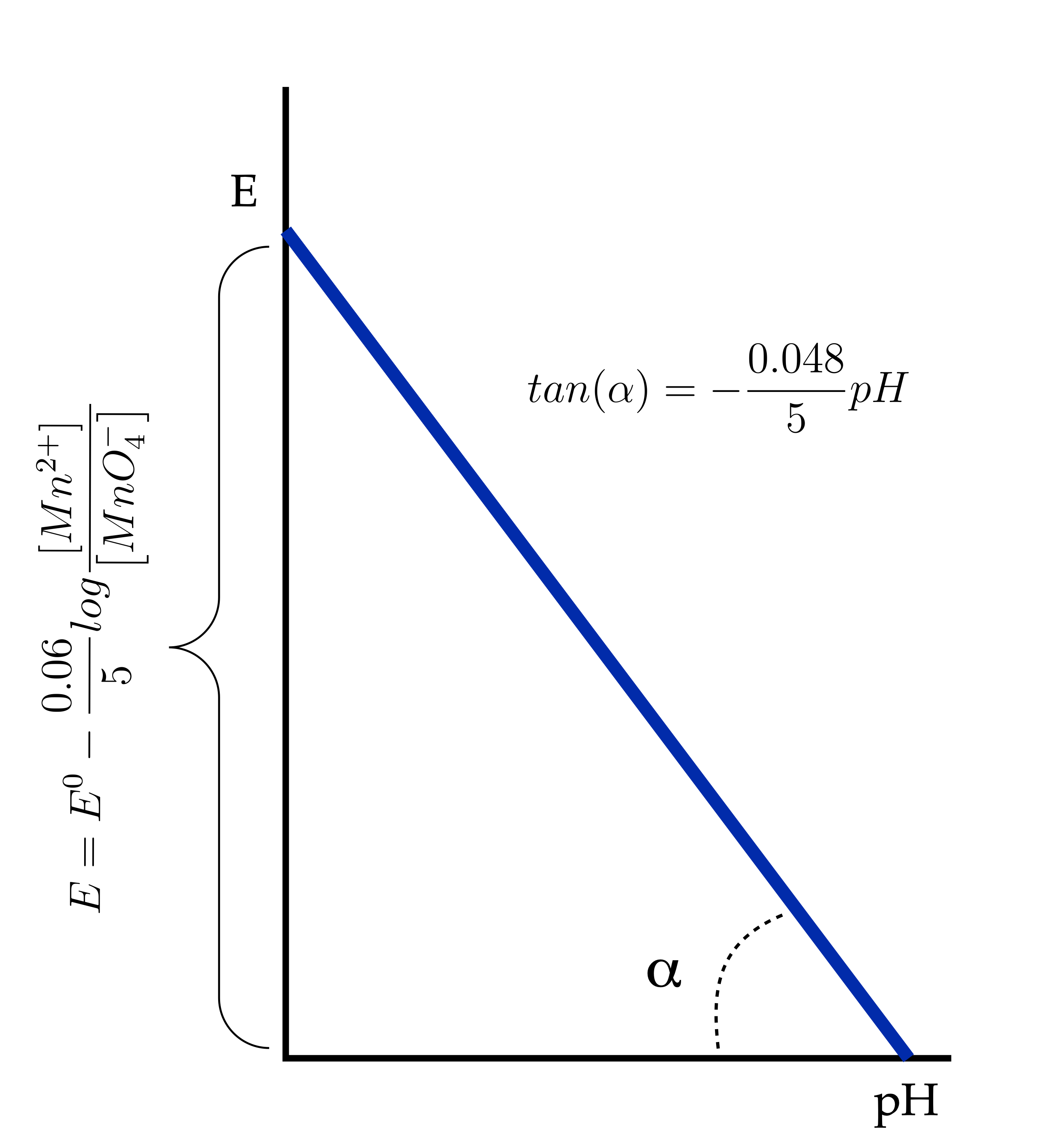
2) In those systems in which oxygen does not vary in the reduction process:
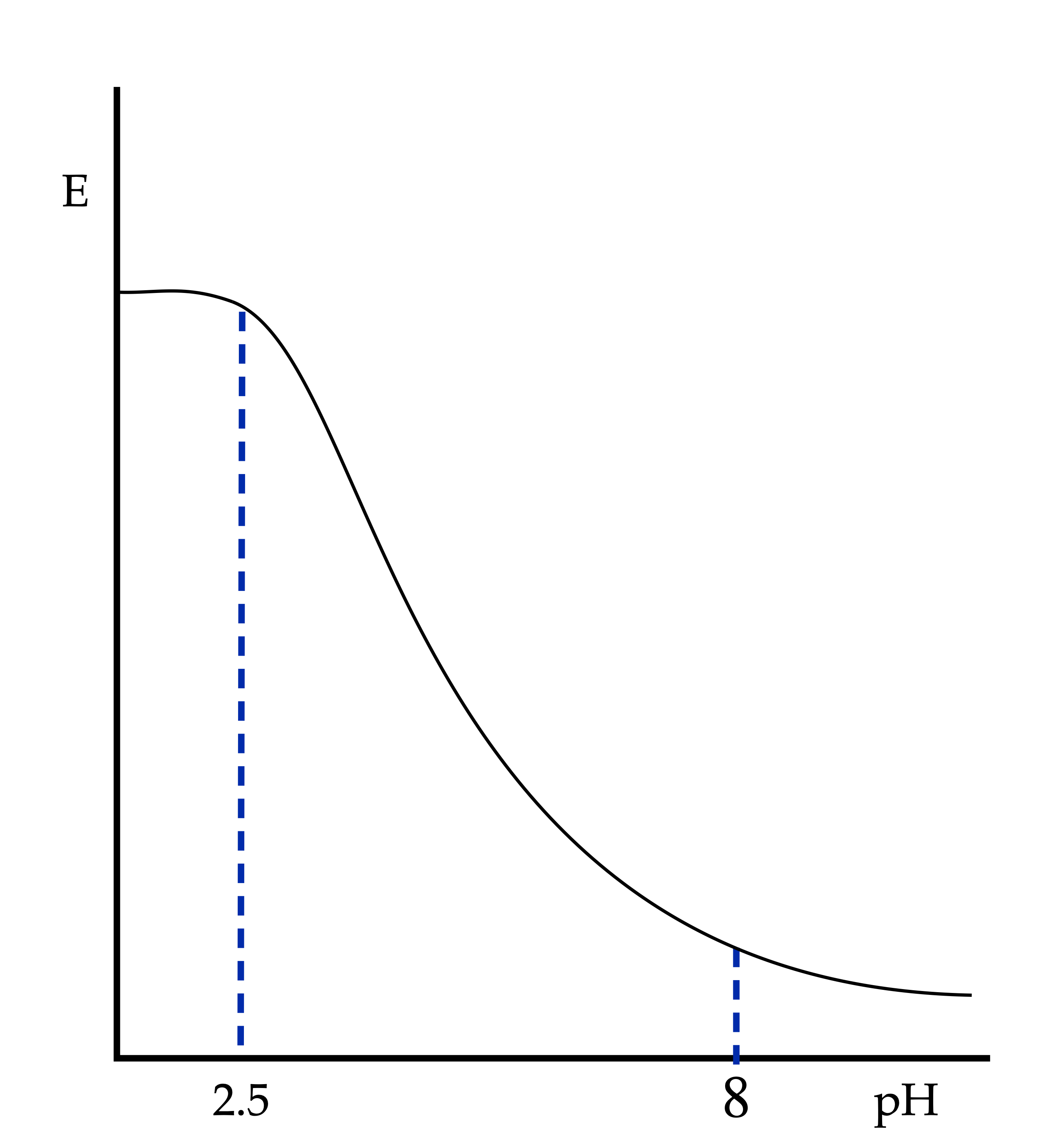
- In the pH range from 0 to 2.5, the HO– concentration is too small to precipitate Fe(OH)3 or Fe(OH)2, so pH does not influence the potential.
- In the pH range between 2.5 and 8, Fe3+ precipitates in the form of Fe(OH)3.
- In the pH range between 8 and 14, Fe2+ precipitates in the form of Fe(OH)2.

Presence of precipitate-forming ions in the medium
Precipitates any of the substances present in the redox equilibrium, will vary constant of said equilibrium and therefore the potential of the reaction:
Applications of normal electrode potentials
[1] It is not rigorously accurate but is fairly close, in some cases it is very erroneous.