Written by J.A Dobado | Last Updated on April 22, 2024
What is a protecting group?
A protecting group is a derivative that blocks a certain functional group from interfering at some stage of a chemical reaction in the synthesis process of a compound. The most commonly used protecting groups in organic synthesis are the protection of alcohol groups (-OH) and carbonyl groups (>C=O).
Examples of protecting groups
Protection of alcohols
| Group {Type} | Protection \ Deprotection | Stable / Incompatible |
Acetate (AcO) CH3COO-R {ester} | CH3COCl / pyridine CH3COOH / H3O+ \ acid or basic hydrolysis | electrophiles, oxidizing agents, mild acidic and/or basic media, NaBH4 / strong acids, bases and organometallic nucleophiles, LiAlH4 |
Benzyl (BnOR) Ph-CH2-OR {ether} | C6H5-CH2-Br / NaH \ hydrogenation | oxidants, bases, nucleophiles, reductants / strong acid, H2 /cat, Na/NH3 |
Trityl (TrO-) (Ph)3C-OR {ether} | (C6H5)3C-Cl / pyridine \ acid medium | bases / acids |
TESO- {silyl ether} | (Et)3Si-Cl / base \ F– or H3O+ | oxidants, nucleophiles /acidsa |
TBDMSO- {silyl ether} | t-Bu(CH3)2Si-Cl / base \ F– or H3O+ | oxidants, nucleophiles /acidsa |
TIPSO- {silyl ether} | (is-Pr)3Si-Cl / base \ F– or H3O+ | oxidants, nucleophiles /acidsa |
TMSO- {silyl ether} | (Me)3Si-Cl / base \ F– or H3O+ | oxidants, nucleophiles /acidsa |
Protection of diols
1,2-diols and 1,3-diols can be protected in the form of acetals (cyclic or acyclic). The most common are isopropylidene derivatives (acetonides) and another possibility are benzylidene derivatives.
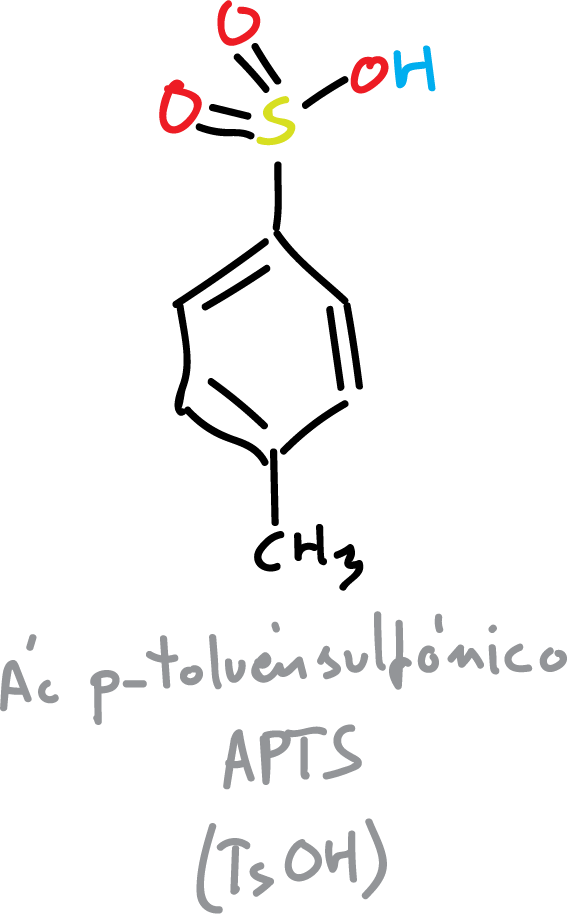
The acetonides will be formed with the corresponding diol plus acetone. An acid catalyst is needed for the reaction to take place, usually para-toluensulfonic acid (PTSA or TsOH).
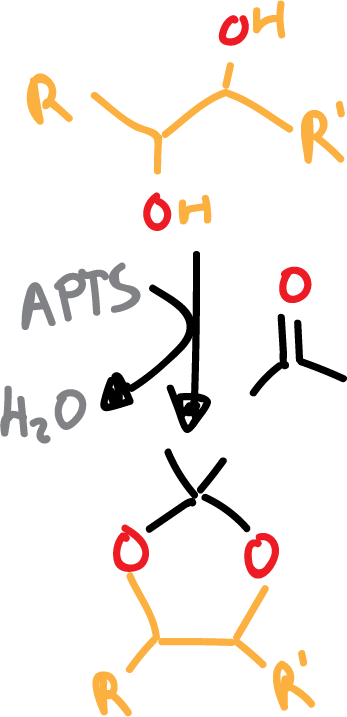
For the reaction to proceed correctly, water must be removed from the medium.
Another possibility in which benzaldehyde in acid medium (acid catalysis) is used instead of acetone is shown in the following scheme:
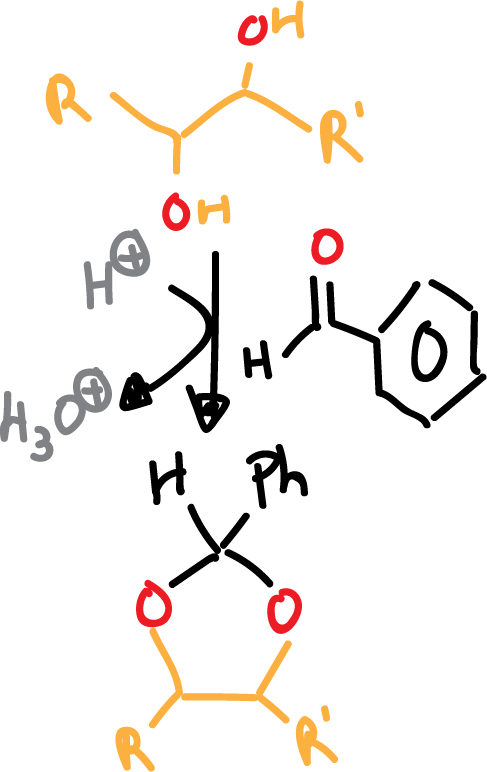
In both cases, the subsequent deprotection step is carried out in aqueous acidic medium. Benzylidene derivatives can also be deprotected by hydrogenolysis (H2/Pd).
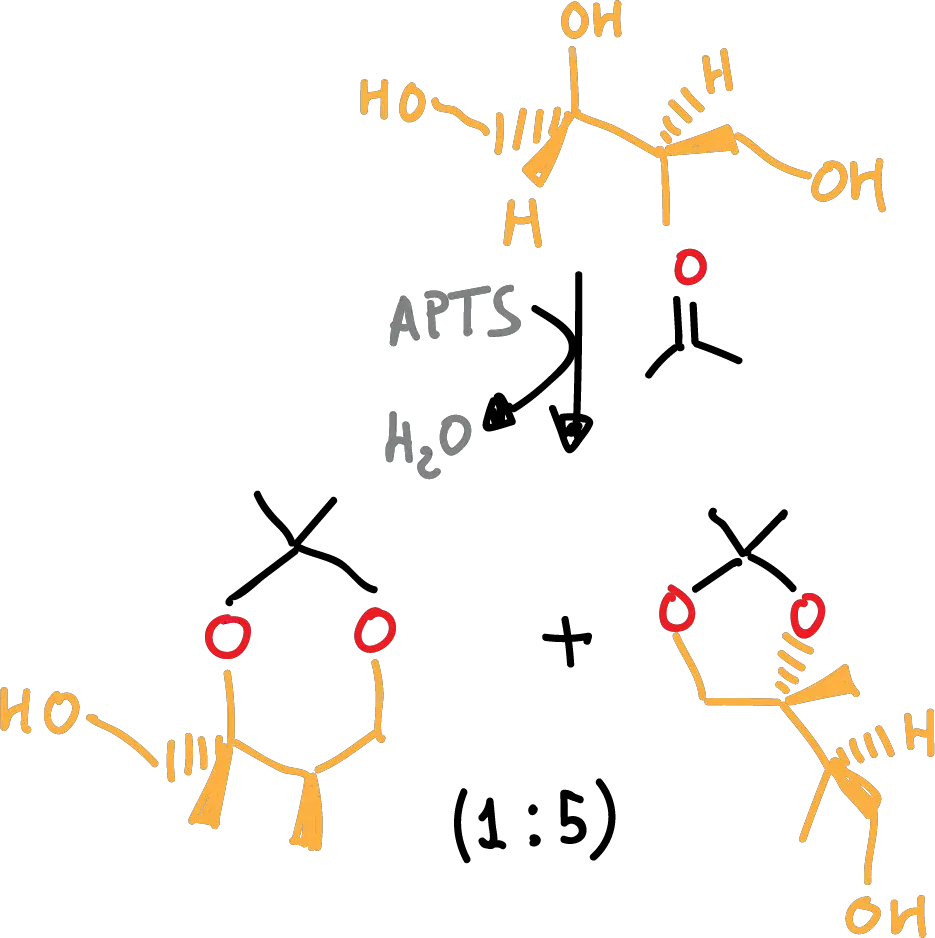
The acetonides preferably form 5-membered rings while the benzylidene derivatives preferably form 6-membered rings.
For example, the following scheme shows the preference of acetonides to form 5-membered rings.
On the other hand, this other example illustrates the preference of benzylidene derivatives to form 6-membered rings.

The most frequent protective groups of 1,2-diols and 1,3-diols are summarized below:
| Group {Type} | Protection \ Deprotection | Stable / Incompatible |
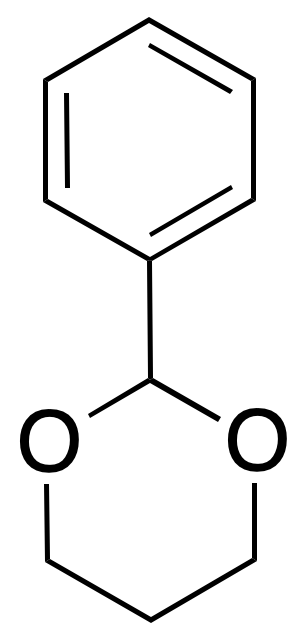 {acetal} {acetal} | Benzaldehyde / mineral acid or Lewis acid. \ hydrogenolysis or acid hydrolysis, I2 / acetone | Bases, nucleophiles, oxidants, reductants / acid medium, halogens, H2 / cat. |
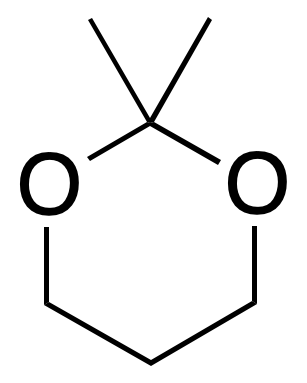 | Acetone / acid \ acid hydrolysis I2 / acetone | Bases, nucleophiles, oxidants, reductants / acid medium |
Protection of aldehydes and ketones
The carbonyl groups (aldehydes and ketones) will be protected when a nucleophile or reducing agent is used. In this way we prevent the carbonyl group from reacting.
- Formation of ketals or acetals: cyclic acetals are usually formalised and the reagent used is ethylene glycol in the presence of acid.
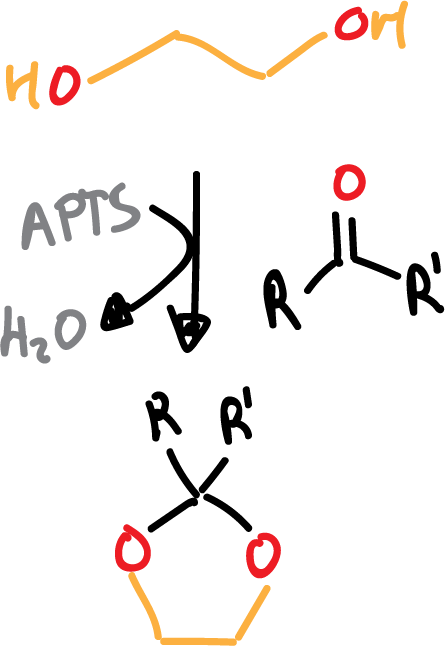
As in the case of glycols, deprotection is carried out in an aqueous acid medium (H2O/H+).
- Formation of 1,3-oxathiolanes: in this case we use 2-hydroxy-1-ethanethiol (HS-CH2-CH2–OH) in the presence of boron trifluoride, BF3 ( Lewis acid) as a reagent.
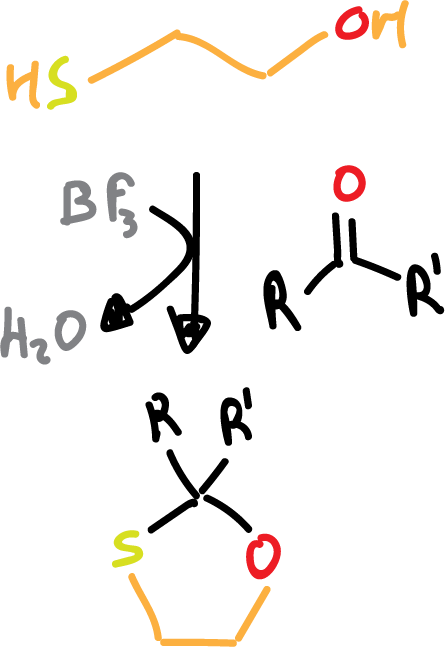
In this case the deprotection is achieved with Raney Nickel which is a reductant.
- Formation of dithioacetals: in this case, we will use the HS-CH2-CH2–SH dithiol in the presence of a Lewis acid.
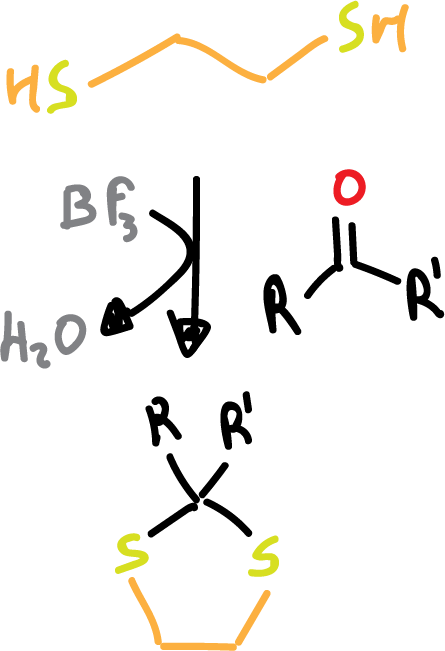
The deprotection is carried out in aqueous medium in the presence of a Cl+, NO+, or Cu2+ source that activates the deprotection. As can be seen, deprotection of these groups can be carried out selectively.
In Table 3, the most common protecting groups of aldehydes and ketones are summarized.
| Group {Type} | Protection \ Deprotection | Stable / Incompatible |
RCH(OR’)2 {cyclic or acyclic acetal} | R’OH / H+ \ acid hydrolysis | bases, nucleophiles, reductants / electrophiles, acids |
RCH(SR’)2 {cyclic or acyclic thioacetal} | R’SH / mineral acid or Lewis acid \ mercuric salts | nucleophilic bases, electrophiles (except MeI) / oxidants, H2/cat, halogens, peracids, MeI |
Protection of amines
Amines are nucleophiles and on the other hand hydrogen has a weakly acidic character and therefore will react with bases.
If either of these two features interfere with the synthetic sequence, the amine must be protected (secondary, R2NH, or primary, RNH2).
There are several possibilities for their protection:
- Formation of carbamates or amides:
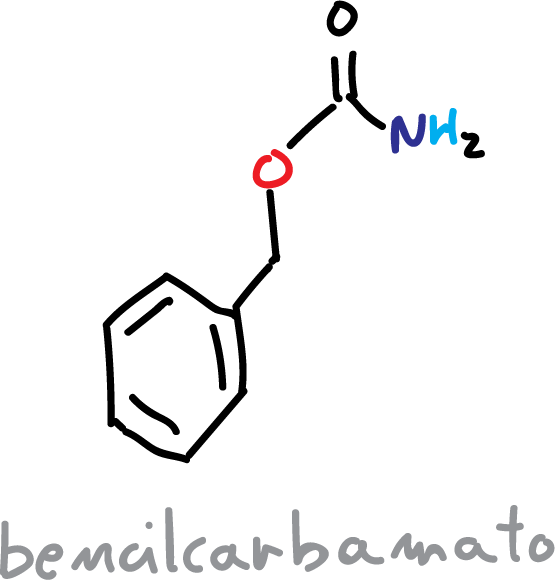
The most frequently obtained carbamates are t-butylcarbamate (BOC) or benzylcarbamate (CBz).
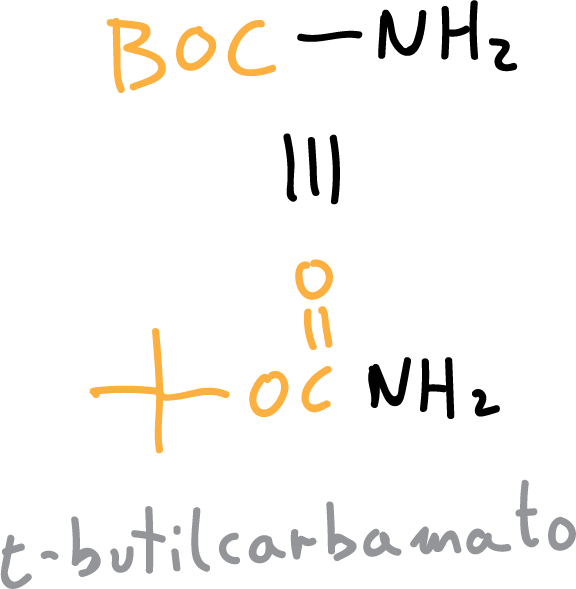
The BOC protection reaction proceeds in a basic aqueous medium, sodium hydroxide (NaOH) or triethanolamine (Et3N) can be used, and proceeds as follows:
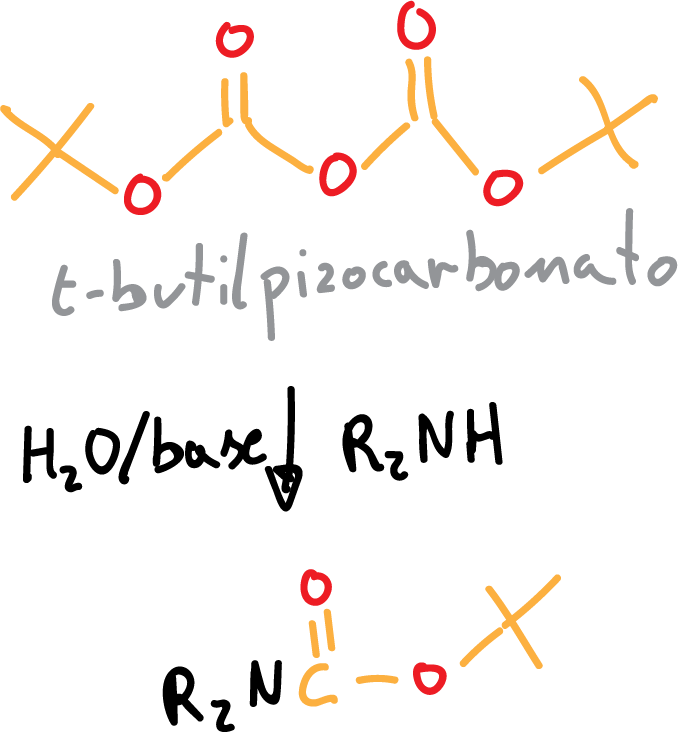
On the other hand, deprotection is performed by using an acid (PTSA, CF3COOH).
If we use CBz, the protective reaction is performed as follows:
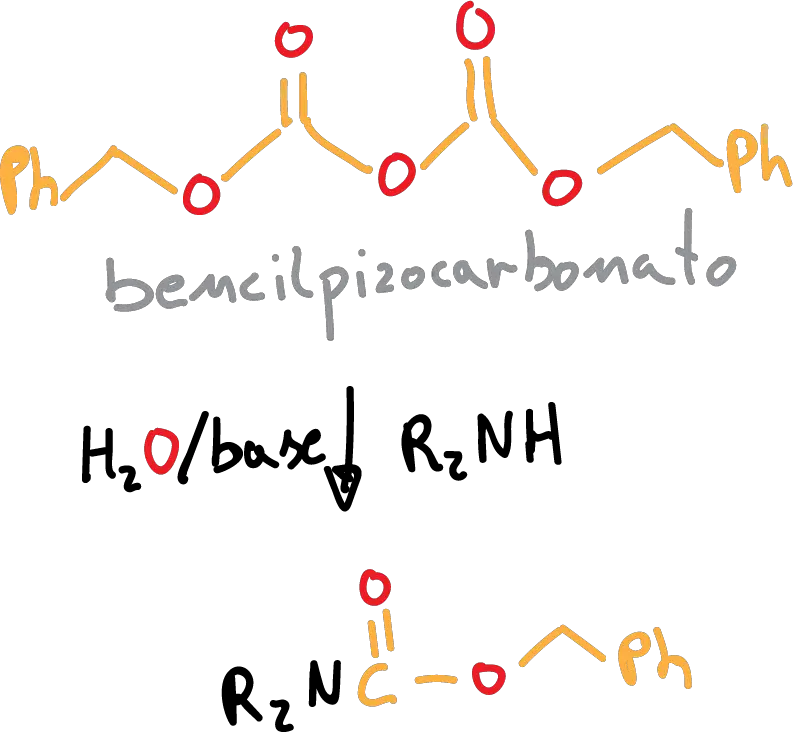
This group can be deprotected by hydrogenolysis (H2/Pd).
- Formation of amides:
Protection of primary amines can be carried out in the form of phthalimides, using phthalic anhydride, as follows:

Deprotection is performed with hydrazine (H2N-NH2) or sodium borohydride (NaBH4) in EtOH/water.
Finally, Table 4 summarizes the most frequent protective groups of amines.
| Group {Type} | Protection \ Deprotection | Stable / Incompatible |
Acetamide AcONHR’ {amides} | Ac2O CH3COCl / base \ hydrolysis with strong acids | Oxidant electrophiles Bases , NaBH4, Hydrogenation / RLi, LiAlH4, LDA |
t-butylcarbamate (BocNHR) RNHOCOt-Bu {carbamate} | With base \ HCl 3 M o CF3COOH | Bases, oxidant, nucleophilic reductants except RLi, RMgX/ strong acids |
Protection of carboxyl groups
Protection of a carbonyl group (-COOH) can be carried out in two ways: either by protecting the -OH, or by protecting the carbonyl >C=O.
The hydroxyl (-OH) is protected in ester form, while the carbonyl (>C=O) is realized in oxazoline form, from the reagent isobutanol-2-amine.
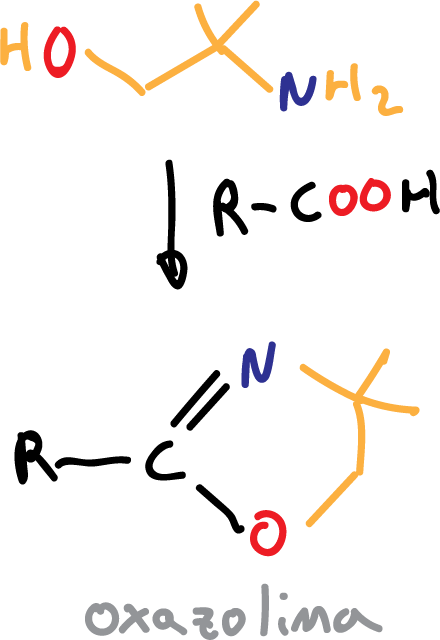
Another reagent that has been used is 2,2-dimethylaziridine.

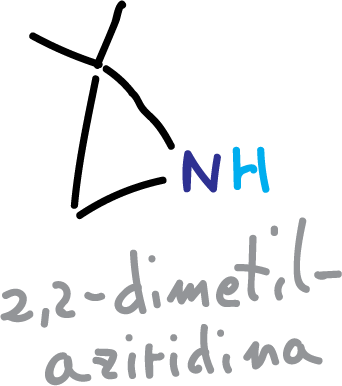
Deprotection is performed in aqueous media (H+/H2O).