Written by J.A Dobado | Last Updated on April 22, 2024
Objective
Students will familiarized with the strict anhydrous conditions involved in the use of Grignard reagents, and in this case for obtaining benzoic acid by the carbonation reaction of a Grignard reagents.
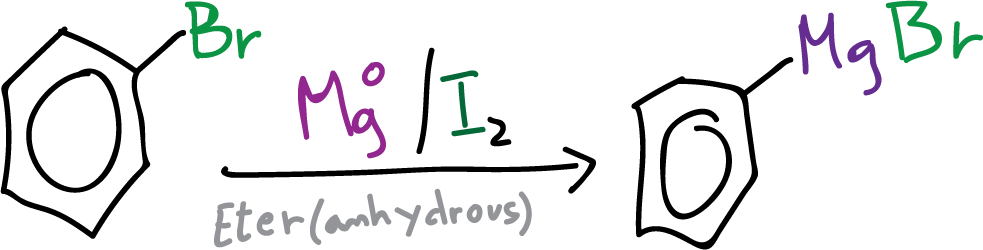
Background
One of the most popular methods of forming C-C bonds in organic synthesis is through the use of Grignard reagents (Nobel Prize in Chemistry for 1912). The formation of these organometallic compounds involves the reaction of an alkyl, vinyl or aryl halide with magnesium and involves a change in the electronic nature of the carbon atom, which changes from electrophile in the halide to strongly nucleophile in the organomagnesium compounds.
R-X + Mg → R-MgX
X = Cl, Br, I
Grignard compounds are highly reactive, they react with water, oxygen, CO2, etc. Therefore, these compounds must be prepared under anhydrous conditions using an inert atmosphere. They have a strongly nucleophilic (and basic) character and produce additions to carbonyl groups, with the formation of a new C-C bond, giving alcohols whose nature depends on the type of carbonyl starting compound used.
A Grignard reagent is considered to be a carbon or carbanion bond (the magnesium salt of an acidic hydrocarbon). However, it is more accurate to consider Grignard reagents as having a highly polar covalent C-Mg bond, rather than an ionic bond between C⊖ and ⊕MgX.
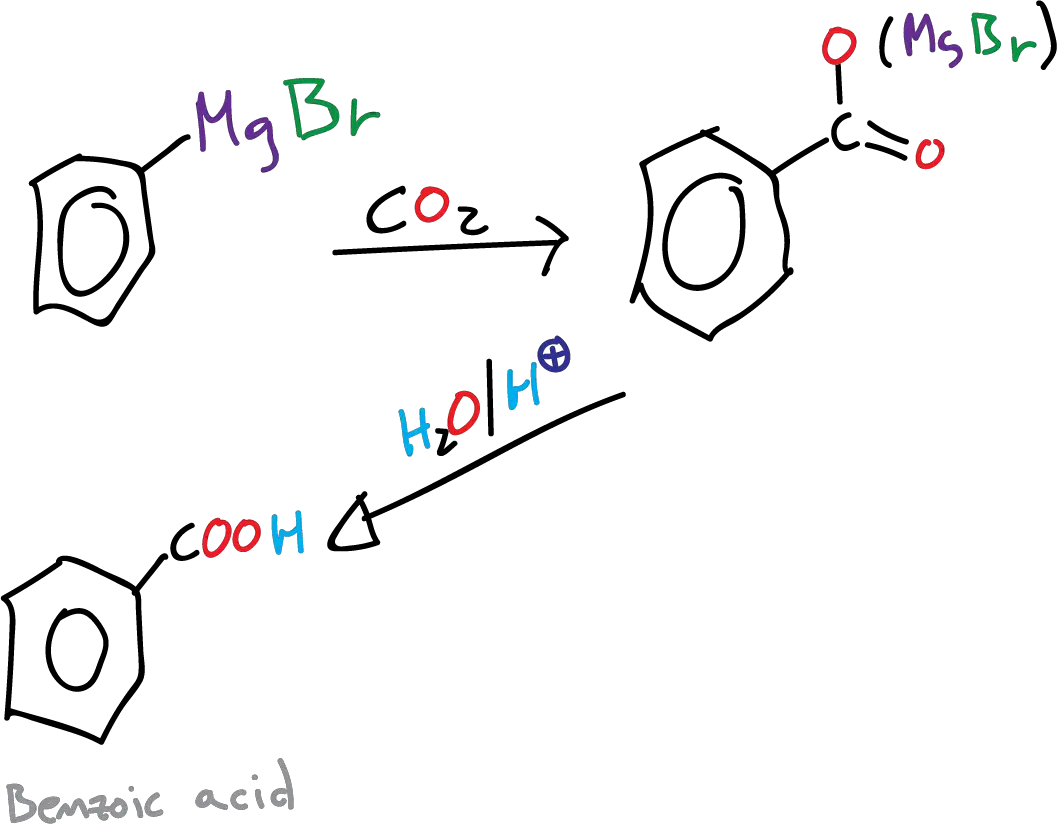
In this experiment, we will proceed to synthesize benzoic acid from bromobenzene by converting the latter into a Grignard reagent (Ph-MgX). The Grignard reagent is then reacted with gaseous carbon dioxide (CO2) to form benzoic acid.
The Grignard synthesis of a carboxylic acid (R-COOH) is achieved by a carbonization step by bubbling carbon dioxide, gaseous CO2, into a solution in THF or diethyl ether of the reagent. In our case, ground dry ice (solid CO2) is used over the Grignard reagent. The advantage of using dry ice is that it acts not only as a reagent but also as a cooling agent.
The reaction proceeds through the formation of an intermediate carboxylate salt, which is then protonated to yield benzoic acid.
In conclusion, the synthesis of benzoic acid from a Grignard reagent is a useful method for the preparation of carboxylic acids. The reaction is also highly selective, with no other major products formed and the yield of the reaction was 80%, which is a good yield for this type of reaction.
Experimental procedure
A) Preparation of Grignard reagent (phenylmagnesium bromide)
In order to carry out this reaction successfully, it is necessary that both the reagents and the material used are completely dry (use an oven for the material if necessary) and work under inert atmosphere conditions.
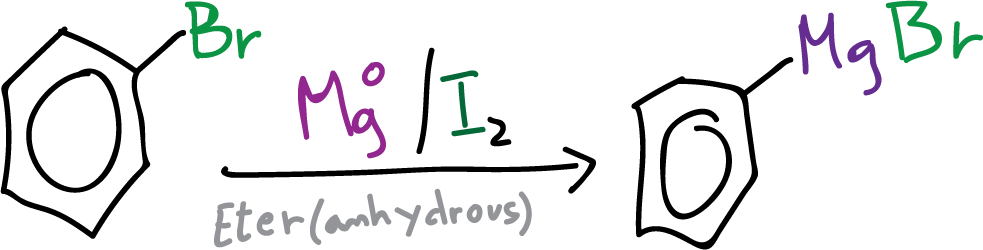
In a 250 ml round bottom flask equipped with a reflux condenser, and an addition funnel, under inert argon atmosphere, 2.4 g of magnesium Mg (in the form of chips or filings) are placed. Subsequently, 30 ml of diethyl ether (anhydrous) are added (THF can also be used).
A small amount of activating agent (usually dibromoethane or iodine) is added and once the magnesium surface is activated (traces of iodine are added to start the reaction at low temperatures without having to resort to an initial heating, since the reaction is then exothermic), slowly add 10 ml of anhydrous bromobenzene.
If the reaction is not started immediately, it is heated in a water bath and removed when the solution begins to boil (discoloration of the iodine and appearance of turbidity is observed).
Since the reaction is exothermic, gentle boiling is allowed to proceed for 30-40 min. At this point, the heating is cut off to control the reaction (because it is exothermic) until the formation of phenylmagnesium bromide.
B) Grignard reagent carbonation
In a beaker of 250 ml, 15.14 g dry crushed ice (solid CO2) is placed. The phenylmagnesium bromide solution prepared in the previous step is poured slowly over it, using magnetic agitation.

A pasty mass is obtained, which is continued stirring until all the solid CO2 has sublimated.
Then, 50 ml of hot water are added, and it is acidified with HCl (dil.), for this way to be able to dissolve the phenylmagnesium salt and to obtain by precipitation the benzoic acid. It is cooled with an ice bath and filtered under vacuum. The benzoic acid thus obtained can be recrystallized from water.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzoic acid | 122.12 | 125 | 249 | 1.08 |
| Argon | 39.95 | -189.2 | -185.7 | - |
| Bromobenzene | 157.01 | -31 | 156 | - |
| CO2 | 44.01 | -78.5 | - | - |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Iodine I2 | 253.81 | 113 | 184 | 4.930 |
| Magnesium | 24.31 | 648 | 1,090 | 1.740 |
| Phenylmagnesium bromide | 181.31 | - | - | - |
| Tetrahydrofuran | 72.11 | -108.0 | 65-67 | 0.89 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzoic acid |   |
| Argon | Non-hazardous |
| Bromobenzene |    |
| CO2 |  |
| Diethyl ether |   |
| HCl |   |
| Iodine I2 |   |
| Magnesium |  |
| Phenylmagnesium bromide |    |
| Tetrahydrofuran |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzoic acid | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
| Argon | XKRFYHLGVUSROY-UHFFFAOYSA-N |
| Bromobenzene | QARVLSVVCXYDNA-UHFFFAOYSA-N |
| CO2 | CURLTUGMZLYLDI-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Iodine I2 | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
| Magnesium | FYYHWMGAXLPEAU-UHFFFAOYSA-N |
| Phenylmagnesium bromide | NIXOIRLDFIPNLJ-UHFFFAOYSA-M |
| Tetrahydrofuran | WYURNTSHIVDZCO-UHFFFAOYSA-N |
Video on the synthesis of benzoic acid from Grignard reagent
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A Three-Step Synthesis of Benzoyl Peroxide
Brenda Her, Alexandra Jones, and James W. Wollack
Journal of Chemical Education 2014 91 (9), 1491-1494
DOI: 10.1021/ed400240k