Written by J.A Dobado | Last Updated on April 22, 2024
Objective
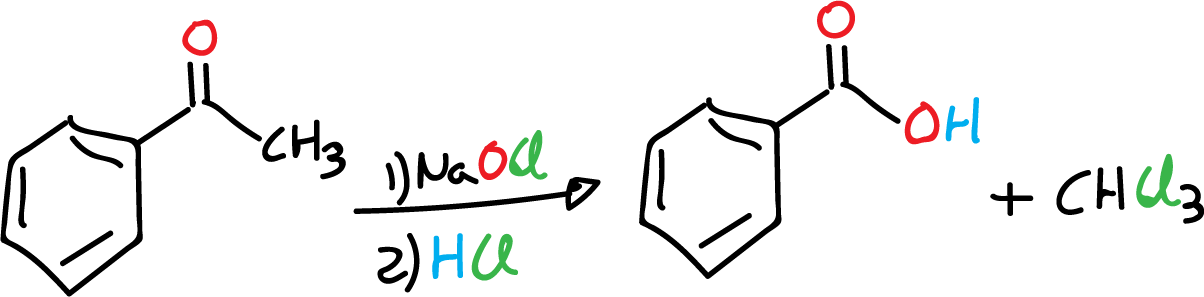
The purpose of this experiment is to carry out a modification of the haloform reaction, using everyday oxidizing chemical reagents such as bleach (sodium hypochlorite, NaOCl).
Background
The haloform reaction is characteristic of compounds that have a methyl group (CH3) adjacent to a carbonyl group (C=O). Families of molecules with this type of functional group grouping are referred to as methylketones. The haloform reaction is carried out using chlorine in a basic medium to give a carboxylic acid. This reaction is used as an analytical test to detect methylketones. In this experiment the process is modified by substituting the chlorine (Cl2) with easily handled commercial bleach (sodium hypochlorite, NaOCl), for the oxidation of methylketone.
Commercial bleach is produced by combining chlorine gas with a solution of sodium hydroxide. The resulting product is a pale yellow liquid with a pungent odor, and it is widely available in stores.
As an oxidizing agent, sodium hypochlorite is capable of removing electrons from other chemicals, thereby causing them to undergo a redox reactions.
Experimental procedure
Weigh 2.5 ml of acetophenone (note the weight in grams), and place it in a 250 ml round bottom flask with stir bar (for magnetic stirring). For each gram of acetophenone used, add 40 ml of commercial bleach (NaOCl 5%) to the flask. Shake the mixture at room temperature while adding 2.5 ml of NaOH 10% solution (for each gram of acetophenone used).
A reflux condenser is attached and the reaction is heated in a bain-marie (water at 70 °C for 20 min of magnetic stirring).
During this heating time, the chloroform (CH3Cl) that is produced evaporates from the mixture. At the beginning of this process, an oily layer of acetophenone can be observed floating on top of the aqueous mixture; at the end of the reaction, the oily phase should not be observed because it has reacted to give the product (benzoic acid).
After heating, cool the reaction to room temperature (use an external ice bath if necessary), remove the reflux condenser and add in small portions 1 ml of acetone, while stirring to remove the remaining NaOCl.
Slowly add HCl (conc.) with a pipette, with stirring, until a significant amount of precipitate has formed. When no more precipitate formation can be detected, mix well and check that the pH of the liquid is 2-3 or less. Cool the mixture in an ice bath to complete crystallization of the benzoic acid product and isolate this product by vacuum filtration.
Wash the product very well with cold water and then let it dry in the vacuum stream for a while. Weigh the dry benzoic acid and calculate the yield and the melting point of the solid.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| Benzoic acid | 122.12 | 125 | 249 | 1.08 |
| Chloroform | 119.38 | -63 | 60.5-61.5 | 1.492 |
| Acetophenone | 120.15 | 19-20 | 202 | 1.03 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| HCl |   |
| NaOH |  |
| Acetone |   |
| Benzoic acid |   |
| Chloroform |   |
| Acetophenone |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| Benzoic acid | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
| Chloroform | HEDRZPFGACZZDS-UHFFFAOYSA-N |
| Acetophenone | KWOLFJPFCHCOCG-UHFFFAOYSA-N |
Video on the oxidation reaction of acetophenone with sodium hypochlorite
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A. M. Van Arendonk and M. E. Cupery, The reaction of acetophenone derivatives with sodium hypochlorite, Journal of the American Chemical Society 53 (1931), no. 8, 3184–3186, DOI: 10.1021/ja01359a506