Written by J.A Dobado | Last Updated on April 22, 2024
What is hydrochloric acid?
Hydrochloric acid is an inorganic mineral acid with the general formula HCl-H2O. It is the simplest chemical system consisting of chlorine and water.
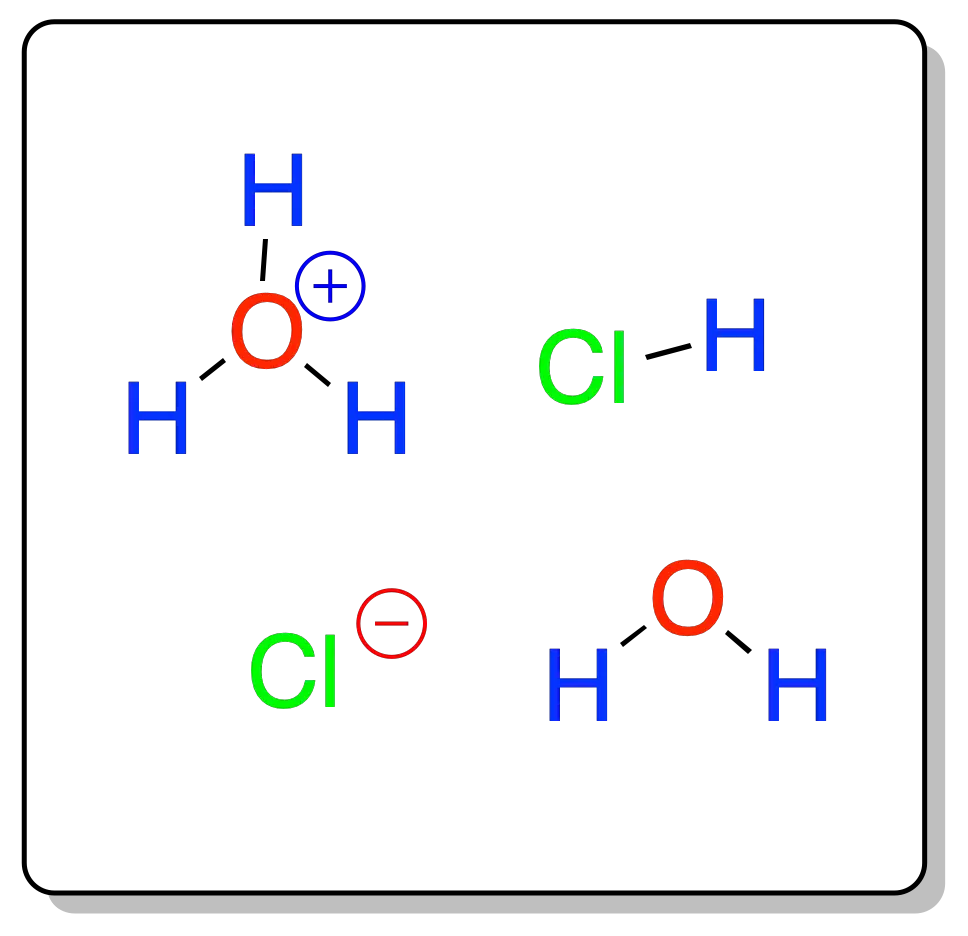
It is formed by dissolving hydrogen chloride gas in water. This solution is composed of hydrogen chloride molecules, water, and ions such as the chloride anion Cl⊖ and the cation oxonium or oxidanium cation H3O⊕. IUPAC does not recommend the use of the name hydronium for this cation.
- HCl + H2O → H3O⊕ + Cl⊖
It is a colorless liquid with an intense odor. It is also called: muriatic acid, spirit of salt, acid of sea salt, marine acid, hydrochloric acid, or salfuman.
This acid is produced naturally in the digestive systems of most animal species, including humans, as a natural component of gastric acid.
Physico-chemical properties
It is very corrosive and acidic and is widely used as a chemical reagent. Being a strong acid, it dissociates completely in water. A concentrated solution of hydrochloric acid has a pH < 1.
It is a monoprotic acid, it can only release one proton H⊕, and the other ion formed Cl⊖ can be used to prepare salts called chlorides, such as sodium chloride.
Monoprotic acids have an acid dissociation constant, Ka, which for strong acids presents a high value. When chlorides, such as NaCl, are added to an aqueous HCl solution, the pH value practically does not change, indicating that the Cl– ion is a remarkably weak conjugate base, and that HCl is almost completely dissociated in aqueous solutions. Therefore, for hydrochloric acid solutions of relatively high concentration, it is assumed that the concentration of H⊕ is equal to that of HCl.
It is one of the least dangerous strong acids to handle and despite its acidity. Its solutions of intermediate concentrations are quite stable (up to 6 M), maintaining their concentrations over time.
Therefore, they make it an excellent acidifying reagent, and also used in acid-base titrations.
It is frequently used in chemical analysis and to digest samples for analysis. Concentrated solutions are also used to dissolve some metals, forming oxidized metal chlorides and hydrogen gas.
The 1:3 (molar ratio) mixture of nitric acid and hydrochloric acid is called aqua regia. The name comes from Alchemybecause this solution dissolved noble metals such as gold and platinum.
Production
Hydrochloric acid is an important chemical reagent in laboratories in industry. Thus, it is used in the production of polyvinyl chloride (PVC) in plastic polymers. Dilute hydrochloric acid is often used as a household descaling agent. It is also used in the food industry as an additive and in the production of gelatine. It is also widely used in leather processing.
It was discovered by the alchemist Jabir ibn Hayyan in 800 AD. Historically, it was called acidum salis and spirits of saltbecause it was produced from rock salt and “green vitriol” (FeSO4) and later from the chemically similar common salt and H2SO4. Hydrochloric acid was first formally described in the 16th century by Libavius. Later, it was used by chemists such as Glauber, Priestley and Davy in their research. Unless pressurized or cooled, hydrochloric acid becomes a gas if the percentage of water is 60% or less.
It is obtained in the laboratory by heating sulfuric acid in the presence of sodium chloride at 150 °C.
H2SO4 + 2NaCl → 2HCl + NaSO4
On an industrial scale, large amounts of hydrochloric acid are produced in the organic chlorination reactions of organic substances with dichlorine.
Another large-scale production method is used by the chlor-alkali industry. This method consists of the electrolysis of a solution of common salt (NaCl), producing chlorine, sodium hydroxide and hydrogen. The chlorine gas obtained reacts with hydrogen to give chemically pure gaseous HCl. The installations are called HCl furnaces because of the exothermic nature of the reaction.