Written by J.A Dobado | Last Updated on April 22, 2024
Objective
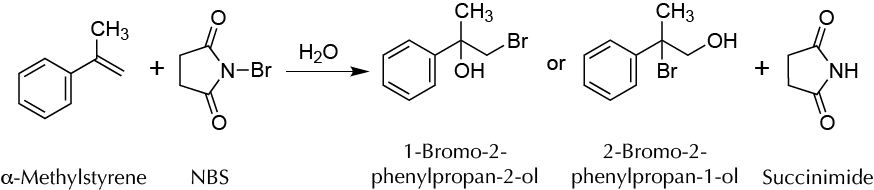
To demonstrate the regioselectivity of electrophilic addition to a double bond. This characteristic (regioselectivity) is not possible in the addition reaction to cyclohexene, because it is symmetrical.
Background
Electrophilic additions to double bonds are ones of the most common as well as the most powerful chemical transformations available. These reactions allow, starting from a relatively simple compound, the addition of a large amount of molecular complexity through a single synthetic step. The reaction chosen here is the bromohydrin formation (addition of bromine and water to the double bond) of styrene. The bromine source employed is the NBS because it is easier to handle than solid elemental bromine (which is liquid and toxic). NBS produces small concentrations of bromine, under reaction conditions that will react with the double bond, forming a bromonium ion. The product of the reaction is succinimide, which can be precipitated from the reaction crude and be removed by filtration. The water of the reaction comes from the commercial acetone containing approximately 5 % of water. Reaction of this intermediate with the bromonium ion may result in the formation of regioisomers according to the attack position.
Experimental procedure
In a flask, place 5.9 g (50 mmol) of α-methyl styrene and 5 ml of acetone. Add 1.025 equivalents of NBS and stir the mixture at r.t. until all the NBS has dissolved. Then stir the solution for an additional 30 min. Remove the solvent by simple distillation (do not use a rotary evaporator due to the low product b.p.) below 60 ºC. Add 25 ml of hexane to the flask, and dry this solution with anhydrous sodium sulfate. Cool the solution in an ice bath, and stir for 15 min. Filter and wash the solid with a small additional amount of hexane. Place the organic extract in a flask and distil hexane (do not use a rotary evaporator because of the low b.p. of products). The residue is the product of the reaction.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| N-Bromosuccimide | 177.98 | 175-180 | - | - |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| α-Methylstyrene | 118.18 | -24 | 165-169 | 0.909 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetone |   |
| Hexane |     |
| N-Bromosuccimide |   |
| Na2SO4 | Non-hazardous |
| α-Methylstyrene |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| N-Bromosuccimide | PCLIMKBDDGJMGD-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| α-Methylstyrene | XYLMUPLGERFSHI-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- B. Andersh, K. N. Kilby, M. E. Urnis, and D. L. Murphy, Regioselectivity in organic synthesis: preparation of the bromohydrin of α-methylstyrene, Journal of Chemical Education 85 (2008), no. 1, 102, DOI: 10.1021/ed085p102