Written by J.A Dobado | Last Updated on April 22, 2024
Objetive
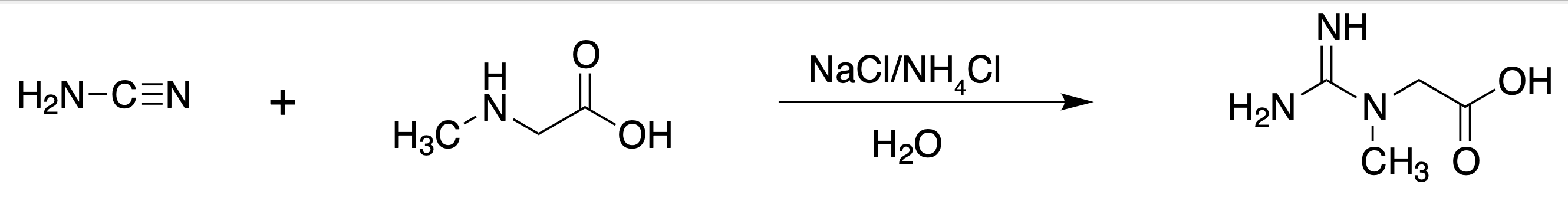
To carry out the synthesis of creatine from the sarcosine molecule (N-methylglycine).
Background
Creatine is a nitrogenous organic acid that occurs naturally in vertebrates, and helps to supply energy to all cells in the body, primarily muscle, playing a key role in muscle energy metabolism.
It is produced in the liver, pancreas, and kidneys and can also be derived from food and dietary supplements. Creatine provides the energy needed for muscle contraction and substantially improves performance in high-intensity exercise because it improves anaerobic capacity and protein synthesis.
Therapeutically, it has been used to treat some types of muscular dystrophy, ocular atrophy, and some types of sclerosis.
Creatine (a dietary supplement) is in high demand in sports, and is neither regulated by the Food and Drug Administration (FDA) nor banned by the International Olympic Committee (IOC). Creatine monohydrate can be synthesized in the laboratory (and commercially) from cyanamide and sarcosine (N-methylglycine).
Experimental procedure
To a r.t. solution of N -methylglycine (sarcosine, 464 mg, 5.2 mmol) in deionized water (1 ml), add NaCl (304 mg, 5.2 mmol). In another flask, to a solution of cyanamide (412 mg, 9.8 mmol) in deionized water (0.26 ml), add two drops of concentrated ammonium hydroxide (approx. 1.4 mmol).
| ¡DANGER! “Cyanamide is extremely irritating and caustic when inhaled, ingested or absorbed through the skin. Do the entire process in a fume hood.” |
Add this solution to the sarcosine and maintain magnetic stirring at r.t. for 1 h. Leave the reaction mixture at r.t. for one week. After a week, vacuum filter the creatine formed. Recrystallize from water.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzamide | 121.14 | 125-128 | - | 1.340 |
| N-Methylglycine | 89.09 | 208-212 | - | - |
| Creatine | 131.13 | 290 | - | 1.330 |
| NaCl | 58.44 | 801 | 1,413 | 2.165 |
| NH4Cl | 53.49 | 340 | - | - |
| NH4OH | 35.05 | -60 | 38-100 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzamide |   |
| N-Methylglycine | Non-hazardous |
| Creatine |  |
| NaCl | Non-hazardous |
| NH4Cl |  |
| NH4OH |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzamide | KXDAEFPNCMNJSK-UHFFFAOYSA-N |
| N-Methylglycine | FSYKKLYZXJSNPZ-UHFFFAOYSA-N |
| Creatine | CVSVTCORWBXHQV-UHFFFAOYSA-N |
| NaCl | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
| NH4Cl | NLXLAEXVIDQMFP-UHFFFAOYSA-N |
| NH4OH | VHUUQVKOLVNVRT-UHFFFAOYSA-N |
Video on biosynthesis of creatine
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A. L. Smith and P. Tan, Creatine synthesis: an undergraduate organic chemistry laboratory experiment, Journal of Chemical Education 83 (2006), no. 11, 1654, DOI: 10.1021/ed083p1654