Written by J.A Dobado | Last Updated on May 2, 2024
Objective
The aim of this experiment (the synthesis of p-nitroaniline) is to familiarize the student with the concept of protective group. In this laboratory experiment, reactions of protection/deprotection of an amino group will be performed.
In this way, reactions such as nitration, which require strongly acidic conditions and to which an amino group is sensitive, will be used.
Background
Aniline cannot be nitrated directly, since the amino group is very sensitive to the acidic conditions of nitration.
Therefore, first the amino group will be protected, then the nitration reaction will be carried out and finally the protecting group will be eliminated to obtain the desired product.
The product obtained in the previous step should be used in each step, so the quantities indicated in the procedure should be adjusted to those obtained in each step.
Experimental procedure
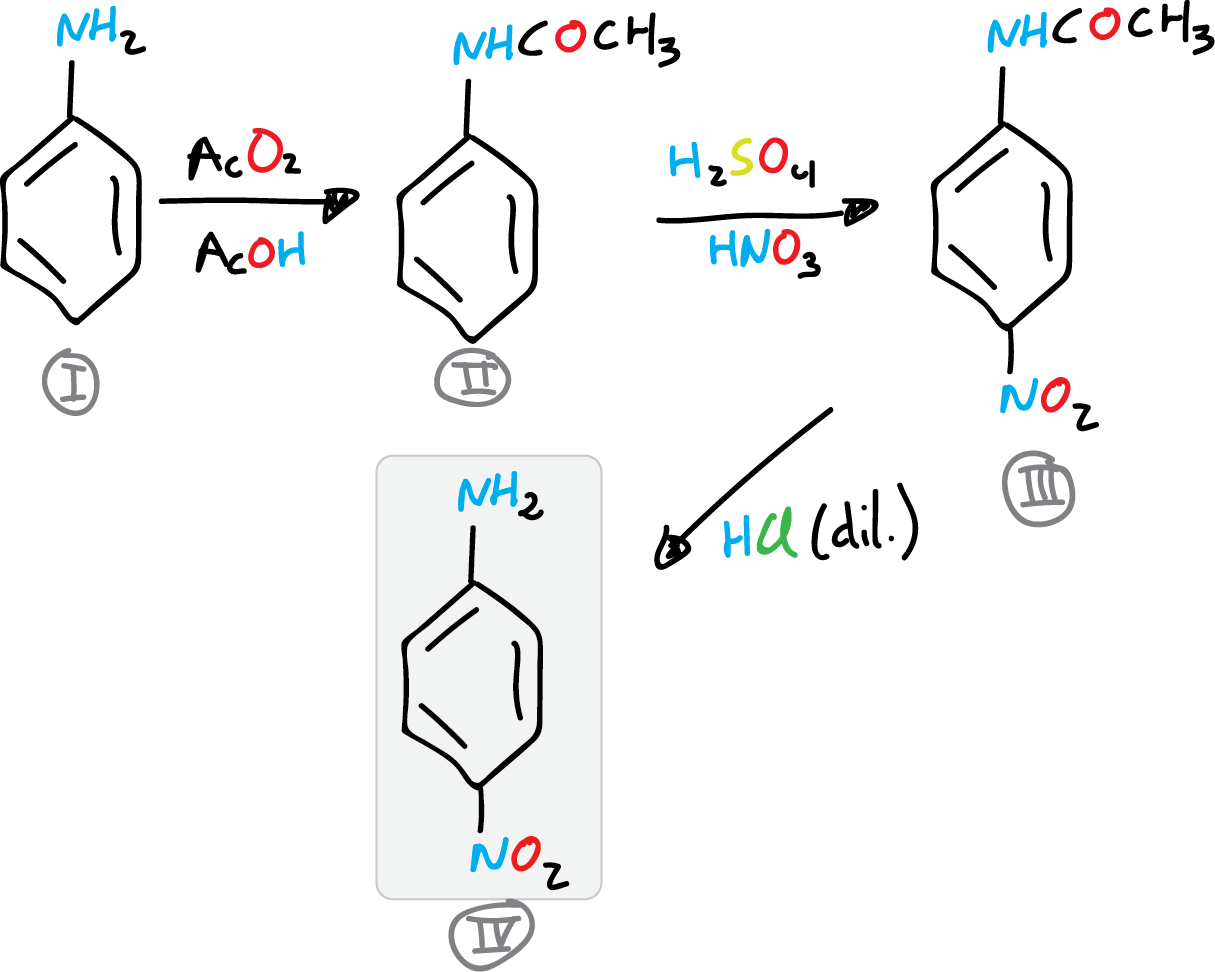
The synthesis of p-nitroaniline will be approached in three steps. In the first one, acetanilide is obtained, then, nitration to p-nitroacetanilide, and finally deprotection to give p-nitroaniline.
A) Preparation of acetanilide
In a 250 ml flask 9 ml (0.1 mol) of aniline, 15 ml of glacial acetic acid and 15 ml of acetic anhydride are placed. A reflux cooler is adapted to the round bottom flask, and the solution is heated to boiling for 10 min. The flask is then allowed to cool.
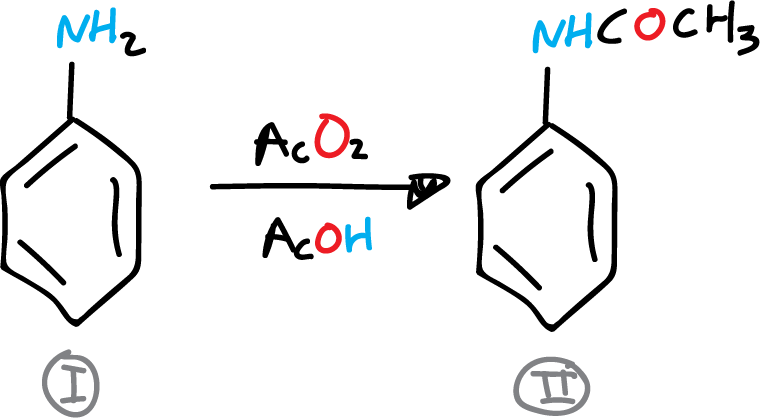
The reaction crude is poured into a beaker with 50 ml water and 40-50 g ice. The mixture is stirred well and the acetanilide crystals are collected by filtration in a Büchner. The product is recrystallized in water, approx. 200 ml, and decolorized with active carbon. It is dried, weighed and the yield is determined.
B) Acetanilide nitration
In a 100 ml beaker 15 ml of concentrated H2SO4 is placed and 6.75 g (0.05 mol) of acetanilide is added, in small portions and with magnetic stirring. As soon as all or nearly all of the acetanilide has dissolved, the beaker is placed in an ice bath and a solution of 6 ml of HNO3 in 6 ml of H2SO4 (conc.) is added using an addition funnel. The mixture is added dropwise, stirring gently and regulating the addition so that the temperature of the reaction mixture does not exceed 35 °C.
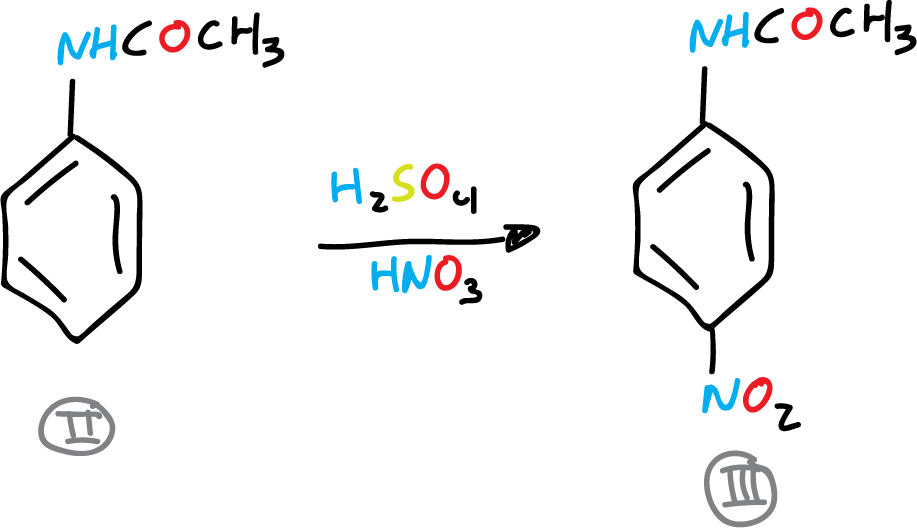
After addition, the beaker is removed from the ice bath and allowed to stand at room temperature for 5 min. The nitrated acetanilide solution is poured into a 600 ml beaker (with approx. 100 ml water and 30 g ice).
The mixture is stirred and the p-nitroacetanilide precipitate is collected by vacuum filtration in a Büchner. In the same Büchner it is washed with two 50 ml portions of cold water pressing well, allowing a stream of air to pass through to dry. It is recrystallized from EtOH. It is dried, weighed and the yield is determined.
| DANGER! “Carry out both nitration (step B) and ammonia addition (step C) in the fume cupboard”. |
C) Preparation of p-nitroaniline
The synthesis of p-nitroaniline concludes with the deprotection of the amino group. For this, the wet p-nitroacetanilide is placed in a 400 ml beaker, and a fine paste is formed with it, adding 100 ml of water and stirring. This mixture is transferred to a 250 ml flask, 35 ml of HCl (conc.) is added and a reflux condenser is adapted to the round bottom flask.
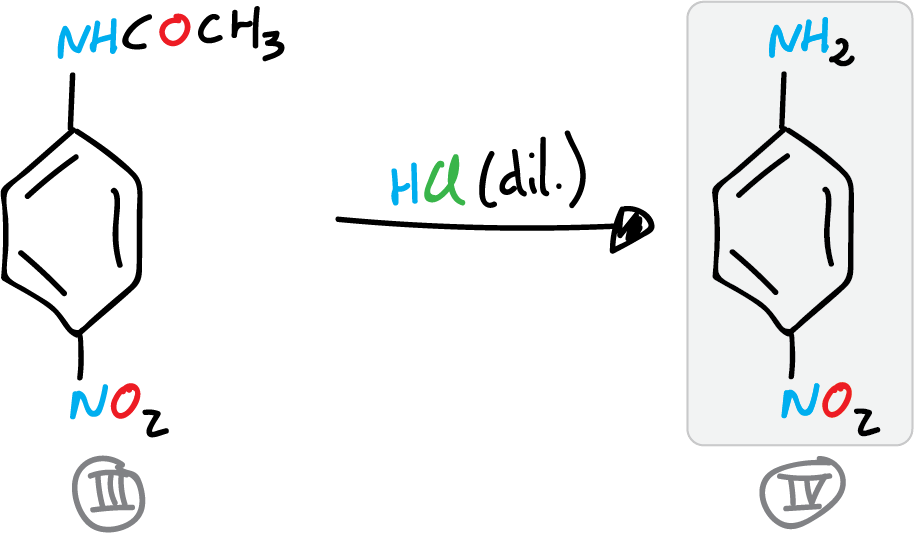
The reaction crude is then heated to boiling point for 35 min. The reaction crude is allowed to cool to room temperature. Once the reaction crude has cooled, it is poured into a 500 ml beaker with approx. 50-75 g of crushed ice. The p-nitroaniline precipitates by alkalinizing the solution by addition of ammonia.
The precipitate is filtered under vacuum in a Büchner, washed with small portions of water. It is recrystallized in water, decolorized with activated charcoal (approx. 0.5 g substance in 40-50 ml water). Dry, weigh and determine the yields (partial and total).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetanilide | 135.16 | 113-115 | 304 | - |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| Aniline | 93.13 | -6 | 184 | 1.022 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| HNO3 | 63.01 | - | 120.5 | 1.413 |
| NH3 | 17.03 | -78 | -33 | 0.590 |
| p-Nitroacetanilide | 180.16 | 213-215 | - | - |
| p-Nitroaniline | 138.12 | 146-149 | 260 | 1.440 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetanilide |  |
| Acetic acid |   |
| Acetic anhydride |    |
| Aniline |     |
| H2SO4 |  |
| HCl |   |
| HNO3 |   |
| NH3 |     |
| p-Nitroacetanilide |  |
| p-Nitroaniline |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetanilide | FZERHIULMFGESH-UHFFFAOYSA-N |
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| Aniline | PAYRUJLWNCNPSJ-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| HNO3 | GRYLNZFGIOXLOG-UHFFFAOYSA-N |
| NH3 | QGZKDVFQNNGYKY-UHFFFAOYSA-N |
| p-Nitroacetanilide | NQRLPDFELNCFHW-UHFFFAOYSA-N |
| p-Nitroaniline | TYMLOMAKGOJONV-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- R. E. Buckles, The use of the Perkin reaction in organic laboratory classes, Journal of Chemical Education 27 (1950), no. 4, 210–211, DOI: 10.1021/ed027p210