Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To produce ethyl iodide at microscale, from EtOH by a nucleophilic substitution reaction.
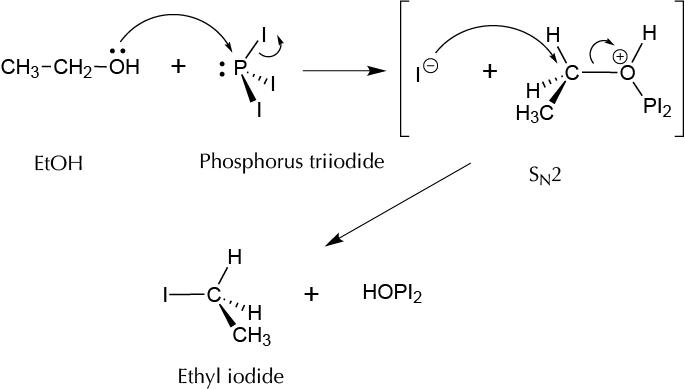
Background
Ethyl iodide is usually prepared in laboratories by the reaction of iodine, red phosphorus, and EtOH. The key step in this synthesis is the formation of the intermediate phosphorus triiodide, which reacts with EtOH to yield the desired product and phosphoric acid as a byproduct.
2P + 3I2 → 2PI3
3CH3−CH2−OH + 2PI3 → 3CH3−CH2 −I + H3PO3
Microscale experimental procedure
In a 10 ml round-bottom flask with an ice-water bath, add 500 mg of red phosphorus, and 5 ml of EtOH. Then, with magnetic stirring, add in 500 mg portions of iodine for approximately 15 min. When the addition is finished, remove from the ice-water bath and fit a water-jacketed condenser with a drying tube, and reflux the mixture for 2 h. After the reflux time, remove the condenser and fit a Hickman column, and separate the final product by distillation, giving a brownish liquid, ethyl iodide, with unreacted free iodine. Transfer the liquid to a conical vial that has been washed with water (several times) to eliminate the remaining EtOH. Then, with several drops of sodium bisulfite (NaHSO3), treat the solution until the brown color disappears. Finally, treat the solution with the same volume of diluted NaOH. Add anhydrous CaCl2, remove the liquid with a Pasteur pipette, and transfer it on a clean conical vial. Fit a clean Hickman column and distill (b.p. = 72 ºC). Weigh and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Ethyl iodide | 155.97 | -108 | 69-73 | 1.950 |
| Iodine I2 | 253.81 | 113 | 184 | 4.930 |
| NaHSO3 | 104.06 | - | - | 1.48 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| PI3 | 411.69 | 61 | - | 4.180 |
| Red phosphorous | 30.97 | 280 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| EtOH |  |
| Ethyl iodide |   |
| Iodine I2 |   |
| NaHSO3 |   |
| NaOH |  |
| PI3 |   |
| Red phosphorous |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Ethyl iodide | HVTICUPFWKNHNG-UHFFFAOYSA-N |
| Iodine I2 | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
| NaHSO3 | DWAQJAXMDSEUJJ-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| PI3 | PZHNNJXWQYFUTD-UHFFFAOYSA-N |
| Red phosphorous | XYFCBTPGUUZFHI-UHFFFAOYSA-N |