Written by J.A Dobado | Last Updated on April 22, 2024
Objetives
Epoxidation of an alkene, in this particular cyclohexene, using an easy-to-handle oxidant such as Oxone®.
Background
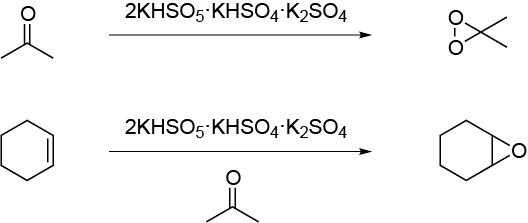
The typical procedure of alkene epoxidation calls for peracids in an organic solvent. One of the most common experimental methods consists of the reaction of peracetic acid in acetic acid. The peracid is generated in situ by the addition of hydrogen peroxide. The proposed green oxidation of one alkene to give the corresponding epoxide is to use the handle itself and save oxidant Oxone® (2KHSO5 · KHSO4 · K2SO4). Epoxidation takes place in a tandem reaction. Oxone® reacts with acetone and sodium bicarbonate to produce dimethyl-dioxirane, and, in the presence of an alkene such as cyclohexene, it is converted to the corresponding epoxide.
Experimental procedure
In a round-bottom flask equipped with a stir bar, prepare a solution of cyclohexene (10 mmol, 0.82 g) in acetone (30 ml). To this solution, add NaHCO3 (4 g, 47.6 mmol) and cool the mixture solution in an ice bath to 0 ºC. In another flask, add dropwise a solution of Oxone® (8.0 g, 13.0 mmol) in water (30 ml). When the addition is complete, remove the mixture from the ice bath, and allow it to reach r.t. while stirring. After 30 min, the reaction is complete. Transfer the reaction mixture to a separatory funnel, and extract with two aliquots of diethyl ether (2 × 25 ml). Combine the organic layers and place back into the separatory funnel and wash with 20 ml of water. Transfer the organic layer to an Erlenmeyer and then dry over anhydrous MgSO4, gravity filter, and transfer to a round-bottom flask. The solvent is removed in a rotary evaporator to give the crude epoxide in nearly quantitative yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Oxone® | 614.78 | - | - | - |
| Cyclohexene | 82.14 | -104 | 83 | 0.779 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
| MgSO4 | 120.37 | 1124 | - | 1.070 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Oxone® |     |
| Cyclohexene |    |
| Diethyl ether |   |
| NaHCO3 | Non-hazardous |
| MgSO4 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Oxone® | HJKYXKSLRZKNSI-UHFFFAOYSA-I |
| Cyclohexene | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
| MgSO4 | CSNNHWWHGAXBCP-UHFFFAOYSA-L |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- W. C. Broshears, J. J. Esteb, J. Richter, and A. M. Wilson, Simple epoxide formation for the organic laboratory using oxone, Journal of Chemical Education 81 (2004), no. 7, 1018–1019, DOI: 10.1021/ed081p1018