Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is to obtain an organic polymer (polystyrene), and for the student to become familiar with the reactions of polymerization initiated by radicals (organic peroxides).
Background
Polystyrene is a polymer that can be prepared by a monomer addition process. The addition reaction is catalyzed by radicals, anions or cations, although the most common processes are radical ones.
In this experiment a radical process with benzoyl peroxide as radical initiator is used. This organic peroxide is a relatively unstable compound that decomposes, between 80 and 90 ºC, with homolytic breakage of the oxygen-oxygen bond.
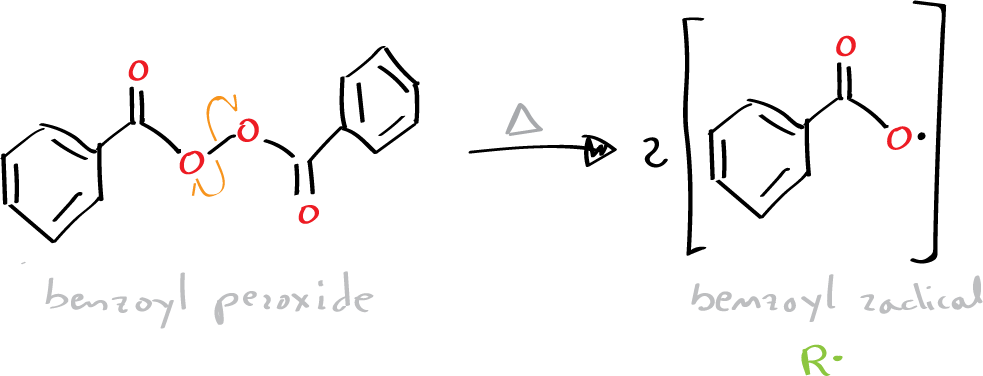
If there is an unsaturated monomer in the medium, the radical will bind to it giving rise to a new radical and a chain reaction is initiated.
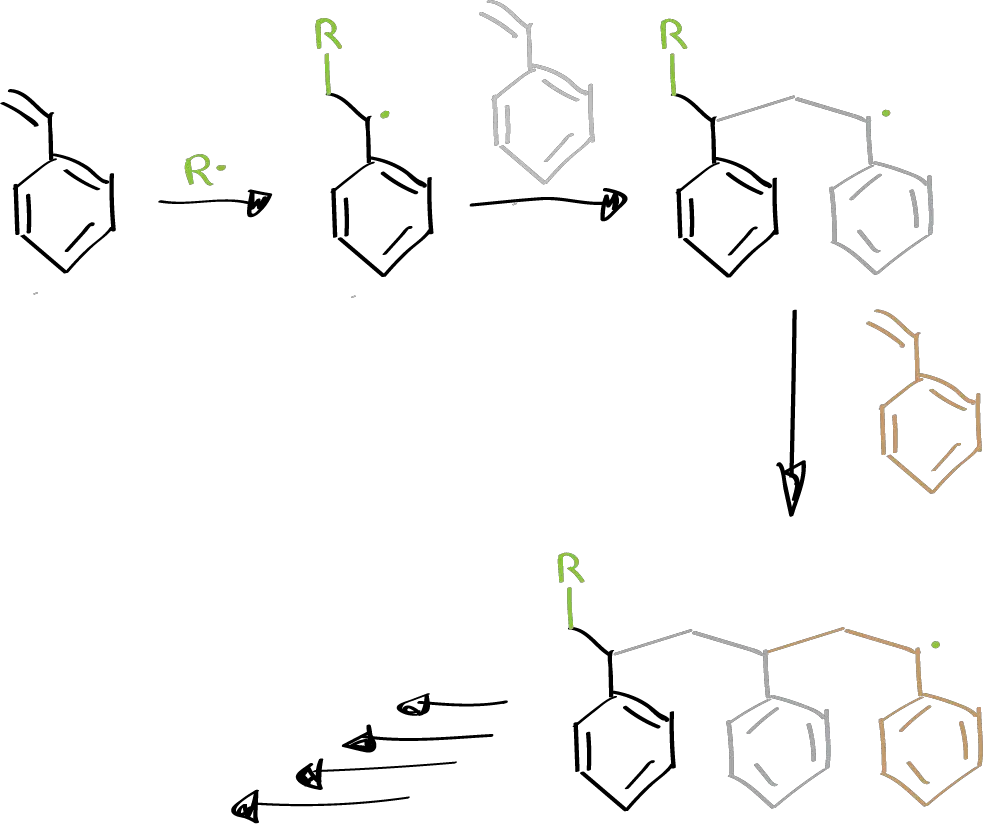
The chain continues to grow and will end by a combination of two radicals, either both polymer radicals, or one polymer radical and one of the initiator, or by abstraction of a hydrogen atom from another molecule.
Experimental procedure
Place 10 ml of styrene in a test tube and add 0.7 g of benzoyl peroxide. It is very important to wash all remaining benzoyl peroxide residues with water, including the disposable substance weighers.
| DANGER! “Benzoyl peroxide is an oxidizing agent and prone to explode both by percussion and by heating or friction. HANDLE WITH EXTREME CAUTION!” |
Heat the mixture over a bath until the mixture has a yellow color. When the color disappears and bubbles begin to form, immediately remove the tube from the bath, as the reaction is exothermic. When the bubbling has subsided, return the tube with styrene to the bath and continue heating until the liquid becomes very sticky.
With a rod take out a thread of material from the test tube. If the strand breaks easily after a few seconds (when it cools down), this indicates that the polystyrene is ready to be poured. If the strand does not break, continue heating the mixture and repeat the above operation until the strand breaks easily.
Pour the sticky liquid into a watch glass. The polystyrene, once cooled, can be peeled off the surface with a spatula.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzoyl peroxide | 242.23 | 105 | - | - |
| Styrene | 104.15 | -31 | 145-146 | 0.906 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzoyl peroxide |   |
| Styrene |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzoyl peroxide | OMPJBNCRMGITSC-UHFFFAOYSA-N |
| Styrene | PPBRXRYQALVLMV-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. W. Armstrong, J. N. Marx, D. Kyle, and A. Alak, Synthesis and a simple molecular weight determination of polystyrene, Journal of Chemical Education 62 (1985), no. 8, 705, DOI: 10.1021/ed062p705