Written by J.A Dobado | Last Updated on April 22, 2024
What is the Fehling’s test?
Hermann Von Fehling discovered in 1849 in a solution (also called reagent Fehling or Fehling’s solution) that could be used for the determination of reducing sugars. Being able to identify the presence of glucose or its derivatives (sucrose, fructose, etc.). It is called Fehling’s test or Fehling’s reaction.
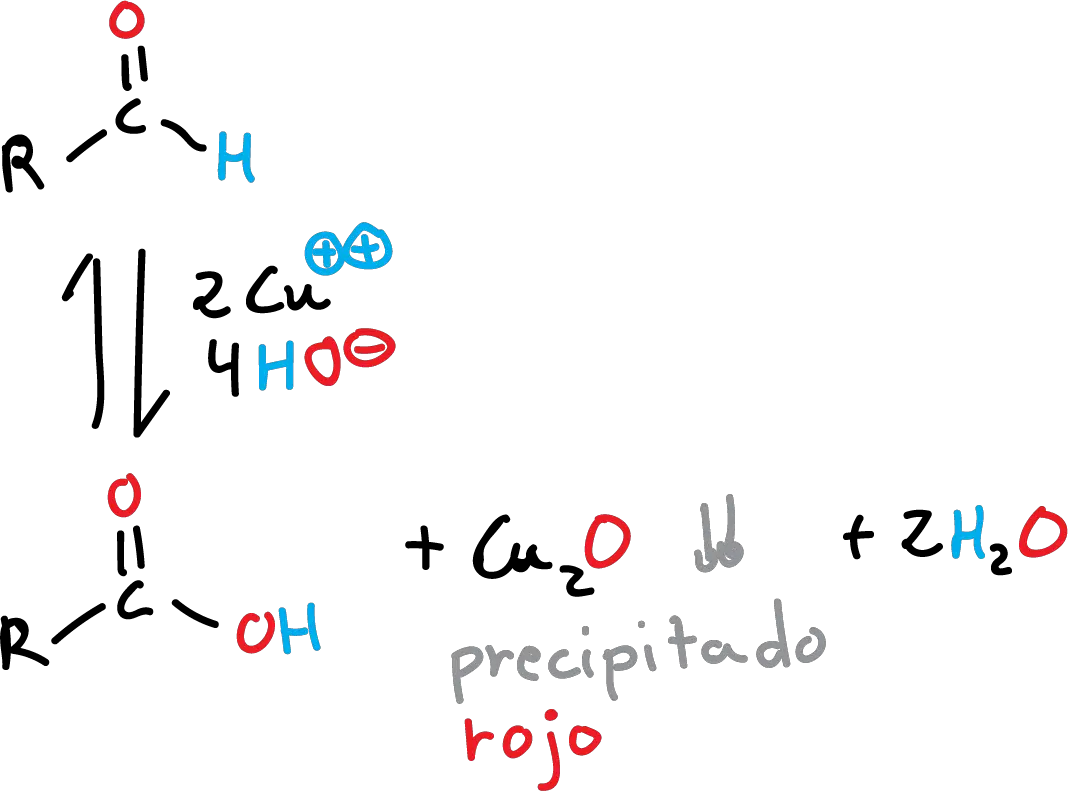
The chemical principle of this test is based on the reaction of oxidation of the copper and the power-reducing sugars (monosaccharides, polysaccharides, aldehydes and some ketones).
The carbonyl group (>C=O) of the aldehydes (has power reducer) and is oxidized to acid, reducing a salt of copper to copper oxide. The reaction is carried out in an alkaline medium to form a precipitate of red that serves to confirm the presence of such groups, carbonyls.
Fehling’s reagent
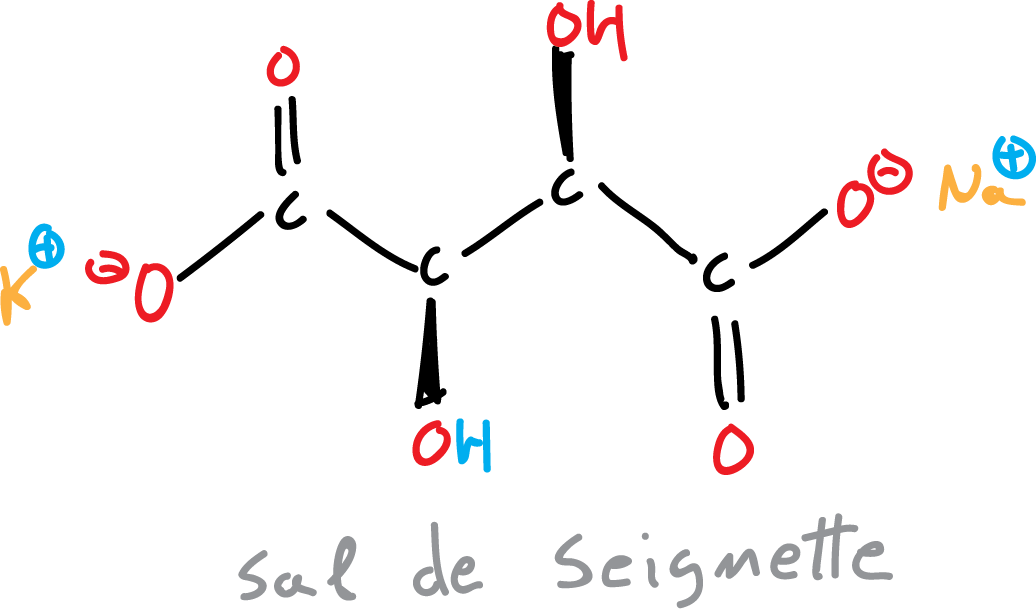
It is prepared at the time of its use by mixing Fehling A (copper solution) with Fehling B (solution of alkaline sodium potassium tartrate) in equal parts.
Forms a complex with the ion cupric which is reduced by the same compounds that reduced the reagent Tollens. If the reaction is positive, it forms a red precipitate of Cu2O.
Fehling’s reagent forms two aqueous solutions: a copper sulfate crystallized and another with Rochelle salt (potassium sodium tartrate).
Both solutions should be stored separately and mixed only at the time of your use.
Example of use
It is used to determine reducing sugars, and demonstrates the presence of glucose in the urine, and also to detect derivatives of glucose, disaccharides such as for example: sucrose or fructose.
Procedure: Prepare two solutions.
- Solution A: dissolve 34.64 g of copper sulfate in 500 ml of water.
- Solution B: 17.6 g of sodium potassium tartrate and 7.7 g of NaOH dissolved in 50 ml of water.
When required, mix 3 ml of each of the solutions. Add to this mix a few drops of the compound liquid of the dissolution of the same, and heated 2 min in a water bath. Used methylene blue for as an indicator of the end point. The appearance of a precipitate red indicates that the test is positive.
Summary
- Essay positive (+): The glucose, lactose and maltose give positive test due to the presence of groups, carbonyls, more exposed that give it character reducer. We observe a precipitate red (copper oxide). The fructosethat presents a group α-hydroxy ketone also gives a positive result.
- Essay negative (-): The sucrose presents groups non-reducing (sugar, non-reducing) and therefore gives a negative result and a colouring blue. It may explain why it is a disaccharide composed of one molecule of glucose and other fructose), the links between the two monomers do not leave free no group reducer.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145