Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To acquaint students with the free-radical halogenation reactions, in particular a bromination using N-bromosuccinimide (NBS).
Background
N-bromosuccinimide (NBS) is used as a highly specific bromination agent both in free-radical substitutions and in electrophilic additions of unsaturated systems, because releases small quantities of bromine.
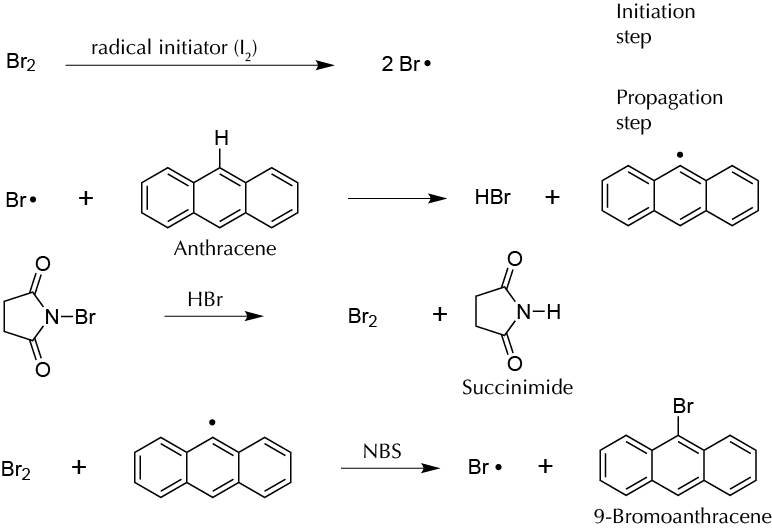
Anthracene can be brominated at position 9 by a radical process. The reaction begins with Br2 formation from NBS. Light or other initiating agents can produce Br·, which attacks anthracene at position 9 to give a 9-anthracenyl radical and HBr. HBr reacts with new molecules of NBS to produce more new Br2 molecules and succinimide. Bromine reacts with the formed 9-anthracenyl radical to give the substitution product and a new bromine radical, which promotes the reaction (propagation step).
Microscale experimental procedure
In a 25 ml Erlenmeyer prepare a solution of 200 mg of I2 in 10 ml of CCl4. Cap the flask containing the mixture with a septum and store in the fume hood for the next step. Mix 50 mg of anthracene (0.28 mmol) 50 mg and NBS (0.28 mmol) with 0.4 ml of CCl4 in a conic vial with a spin vane. With a Pasteur pipette, add 1 drop of the I2/CCl4 solution. Fix a water-jacketed condenser with a dry tube and reflux for 1 h. A brownish color will appear and a solid will form, corresponding to succinimide. After reflux, cool the mixture to r.t. Filter the succinimide under vacuum with a Hirsch funnel, and then wash the solid with cold CCl4 (2 × 1 ml). Transfer the organic solution to a tared 10 ml round-bottom flask, and eliminate the solvent under vacuum (rotary evaporator) to get a greenish yellow solid corresponding to 9-bromoanthracene. Once the solid is dry, weigh and calculate the yield. Purify the final product by recrystallization in a Craig tube in 95% EtOH (m.p. = 101-103 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Anthracene | 178.23 | 210-215 | 340 | - |
| Br2 | 159.81 | 7.2 | 58.8 | - |
| CCl4 | 153.82 | -23 | 76-77 | 1.594 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| HBr | 80.91 | -87 | -67 | 2.140 |
| Iodine I2 | 253.81 | 113 | 184 | 4.930 |
| Succinimide | 99.09 | 123-125 | 285-290 | - |
| 9-(Hydroxymethyl)anthracene | 208.26 | 162-164 | - | - |
| 9-Bromoanthracene | 257.13 | 97-103 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Anthracene |   |
| Br2 |    |
| CCl4 |   |
| EtOH |  |
| HBr |    |
| Iodine I2 |   |
| Succinimide | Non-hazardous |
| 9-(Hydroxymethyl)anthracene | Non-hazardous |
| 9-Bromoanthracene | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Anthracene | MWPLVEDNUUSJAV-UHFFFAOYSA-N |
| Br2 | GDTBXPJZTBHREO-UHFFFAOYSA-N |
| CCl4 | VZGDMQKNWNREIO-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| HBr | CPELXLSAUQHCOX-UHFFFAOYSA-N |
| Iodine I2 | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
| Succinimide | KZNICNPSHKQLFF-UHFFFAOYSA-N |
| 9-(Hydroxymethyl)anthracene | JCJNNHDZTLRSGN-UHFFFAOYSA-N |
| 9-Bromoanthracene | ZIRVQSRSPDUEOJ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- N. M. Weinshenker, 9-Bromoanthracene, Organic Preparations and Procedures 1 (1969), no. 1, 33–34, DOI: 10.1080/00304946909458344