Written by J.A Dobado | Last Updated on April 22, 2024
Objective
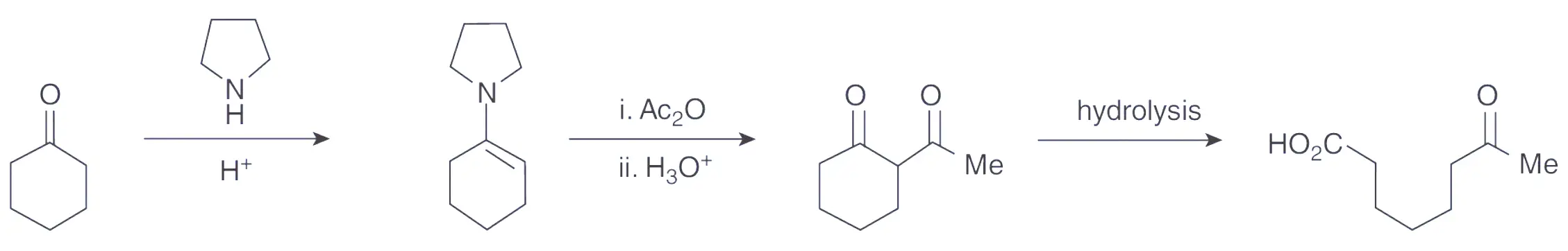
Learn how to prepare an enamine from cyclohexanone and a secondary amine and perform a subsequent acetylation reaction, and finally an hydrolysis to 7‐oxooctanoic acid.
Background
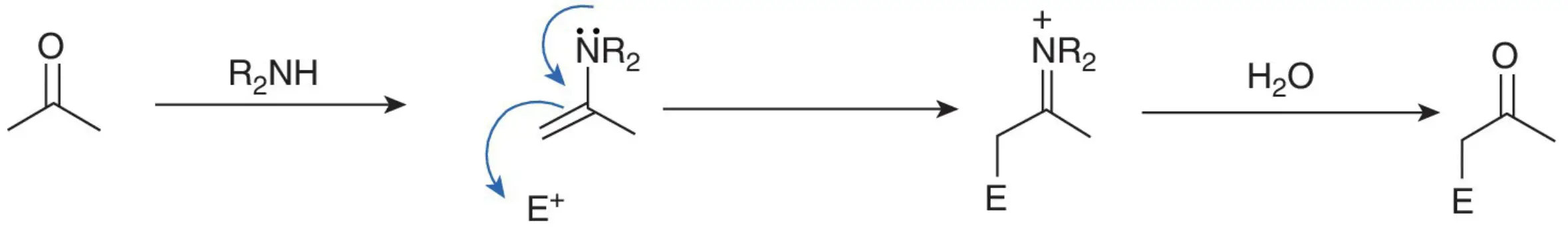
Enamines, which can be easily prepared from carbonyl compounds, exhibit nucleophilic behavior at their β-carbon atom, making them susceptible to alkylation or acylation with appropriate electrophilic reagents.
While organometallic compounds and carbanions are not the only species possessing a nucleophilic carbon atom, the delocalization of the nitrogen lone pair through the double bond to the β-carbon results in the nucleophilic nature of enamines. Upon alkylation or acylation, an enamine forms an iminium ion, which can be reverted to a carbonyl compound upon hydrolysis.
The objective of this experiment is to demonstrate the acetylation of cyclohexanone via its pyrrolidine enamine. The first step involves the conversion of cyclohexanone into its corresponding enamine, 1-pyrrolidinocyclohexene, through the reaction with pyrrolidine in the presence of an acid catalyst. The reaction takes place in boiling toluene and a Dean and Stark apparatus is used to eliminate the water formed during the reaction.
Upon formation, the enamine is not isolated but immediately reacted with ethanoic anhydride to undergo acetylation. After the reaction, an aqueous work-up is carried out to hydrolyze the material to obtain 2-acetylcyclohexanone. This compound, which is a 1,3-diketone, exists in a mixture of keto and enol forms. The percentage of enol content can be estimated through analysis of the 1H NMR spectrum. Subsequently, the 2-acetylcyclohexanone is purified by vacuum distillation. In an optional step, 2-acetylcyclohexanone can be hydrolyzed to produce 7-oxooctanoic acid, which serves as an example of a general route to keto fatty acids.
Experimental procedure
A) Preparation of 2‐acetylcyclohexanone
To carry out this procedure, take a 100 mL round-bottom flask and add cyclohexanone, pyrrolidine, toluene-4-sulfonic acid, a boiling chip, and 40 mL toluene. Attach the Dean and Stark apparatus to the flask and fit the reflux condenser, which should be protected with a drying tube, to the top of the apparatus. Heat the flask until the toluene boils vigorously, allowing the water formed during the reaction to collect in the trap. Maintain the solution at reflux for an hour. Meanwhile, prepare a solution of ethanoic anhydride in 10 mL toluene for later use.
After one hour, allow the solution to cool and remove the Dean and Stark apparatus. Then, fit the still head and thermometer and reassemble the condenser with a receiver and receiving flask for distillation. Distill off the remaining pyrrolidine and water by heating the flask, and continue the distillation until the temperature at the still head reaches 108-110 ºC. Remove the heat and allow the flask containing a toluene solution of the enamine to cool to room temperature.
Next, remove the still head and add the toluene solution of the enamine to the ethanoic anhydride solution. Stopper the flask and let it stand at room temperature for at least 24 hours. Then, add 5 mL water to the flask and fit a reflux condenser. Heat the mixture under reflux for 30 minutes before cooling it to room temperature and transferring it to a 50 mL separatory funnel containing 10 mL water. Shake the funnel, separate the layers, and wash the organic layer with 3×10 mL hydrochloric acid (3 M), followed by 10 mL water. Dry the organic layer over MgSO4, filter off the drying agent, and concentrate the filtrate on a rotary evaporator. Finally, transfer the residue to a small distillation set and distill the material under reduced pressure (ca. 15 mmHg) using a water aspirator or vacuum pump. Record the boiling point, yield, and IR and 1H NMR (CDCl3) spectra of the product.
B) Preparation of 7‐oxooctanoic acid
In a 50 mL round-bottom flask, weigh 1.40 g (10 mmol) of 2-acetylcyclohexanone. Add 3 mL of potassium hydroxide KOH solution and heat the mixture on a boiling water or steam bath for 15 minutes. After cooling, add 30 mL of water and concentrated hydrochloric acid dropwise to the solution until the pH reaches about 7-8. Transfer the resulting solution to a separatory funnel and extract it with 2×5 mL diethyl ether. Discard the ether extracts and acidify the aqueous phase with concentrated hydrochloric acid until the pH reaches 1. Extract the product with 3×10 mL chloroform CHCl3 and dry the combined chloroform extracts over MgSO4. Filter off the drying agent and concentrate the filtrate on a rotary evaporator. If desired, distill the product in a short-path distillation apparatus under vacuum (ca. 4 mmHg). Record the boiling point, yield, and the IR and 1H NMR (CDCl3) spectra of the final product.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2