Written by J.A Dobado | Last Updated on April 22, 2024
Objective
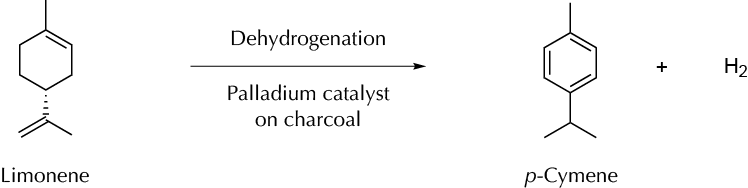
Synthesis of a bulk chemical (p-cymene) from a renewable feedstock (limonene).
Background
D-Limonene is the major constituent of citrus peel oils (90–95 %). Some 50,000 tons of limonene are produced annually by the citrus industry, this being an available renewable feedstock that can be used as the starting material for preparing several products and intermediates. Perhaps the most notable is p- cymene, which has applications in the fragrance and pharmaceutical industries as a solvent, and as a starting material for poly(ethylene terephthalate) (PET), which is a widely used polymer that can be prepared by the direct esterification of terephthalic acid and ethylene glycol or by transesterification of dimethyl terephthalate with ethylene glycol. In both cases, the starting materials are currently petroleum derivatives synthesized from p-xylene by oxidation. First, p-xylene is oxidized to produce terephthalic acid (TA), which is then esterified to dimethylterephthalate (DMT). This transformation can be performed by a two-step sequence or in a one-step reaction in which the oxidation is conducted using a cobalt catalyst in the presence of MeOH. However, it is also possible to prepare terephthalic acid by the oxidation of p-cymene, which in turn can be produced from the dehydrogenation of limonene. In this experiment, the intermediate p-cymene is prepared from limonene, which is isolated from orange peels.
Experimental procedure
A) Isolation of limonene
Grate the citrus fruit rind using the finest texture of a common cheese grater. Care must be taken during the grating of the fruit. It is essential to grate only the flavedo, the colored portion of the peel, avoiding abrading the albedo, or white portion of the inner peel. It is also essential to avoid abrading the pulp to avoid excessive water contamination. Place approximately, 2.5 g of the finely grated peel into a suitable separatory funnel. The rind should then be extracted three times with 7 ml portions of pentane for 10 min intervals, being certain to frequently vent the funnel (pentane is used for the extraction as opposed to the higher boiling hexane because pentane could be more readily removed by evaporation without risking oxidation of the terpene extracts). The combined extracts are then dried over anhydrous sodium sulfate, Na2SO4 (approximately 1 g) for 15 min. Filter the resulting solution to ensure complete removal of the sodium sulfate. Transfer the extract to a tared 50 ml beaker, and remove the solvent over a low heat (approximately 35 ºC), using a very gentle stream of nitrogen to avoid evaporation of the volatile components of the citrus essential oils. After evaporation of the pentane, weigh the essential oils and calculate the yield.
B) Transformation of limonene onto p-cymene
Place limonene (20 ml, 0.125 mol) in a 3-necked 100 ml round-bottom flask fitted with a condenser with an oil trap. One neck is fitted with a Suba-Seal® through which nitrogen gas was passed (through a syringe needle). The final neck is fitted with a glass stopper, through which samples are taken. The limonene is heated, with magnetic stirring to 100 ºC, and 2 ml of limonene should be withdrawn by glass pipette. Pd on charcoal (0.1 g of 5 % wt) is carefully added to the flask through the side arm via a glass funnel; the residue remaining in the glass funnel is washed into the flask using the 2 ml of limonene previously withdrawn. The reaction is carried out under a nitrogen atmosphere at 100 ºC for 3 h. At the end of reaction, allow the reaction mixture to cool before being filtered and weighed.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| N2 | 28.01 | -210 | -196 | 0.970 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| Pd(C) | 106.42 | - | - | - |
| Pentane | 72.15 | -130 | 36.1 | 0.626 |
| R-(+)-Limonene | 136.23 | - | 176-177 | - |
| p-Cymene | 134.22 | -68 | 177 | 0.857 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| N2 |  |
| Na2SO4 | Non-hazardous |
| Pd(C) | Non-hazardous |
| Pentane |     |
| R-(+)-Limonene |    |
| p-Cymene |     |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| N2 | IJGRMHOSHXDMSA-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| Pd(C) | |
| Pentane | OFBQJSOFQDEBGM-UHFFFAOYSA-N |
| R-(+)-Limonene | XMGQYMWWDOXHJM-JTQLQIEISA-N |
| p-Cymene | HFPZCAJZSCWRBC-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. C. Smith, S. Forland, E. Bachanos, M. Matejka, and V. Barrett, Qualitative Analy- sis of Citrus Fruit Extracts by GC/MS: An Undergraduate Experiment, The Chemical Educator 6 (2001), no. 1, 28–31, DOI: 10.1007/s00897000450a
- D. M. Roberge, D. Buhl, J. P. M. Niederer, and W. F. Holderich, Catalytic aspects in the transformation of pinenes to p-cymene., Appl. Catal., A 215 (2001), no. 1-2, 111–124, DOI: 10.1016/S0926-860X(01)00514-2