Objective
To learn how different organic compounds can be isolated according to their acid-base properties, which change their solubility in organic and aqueous solvents, using the liquid-liquid extraction technique.
 |  |
 |  |
Background
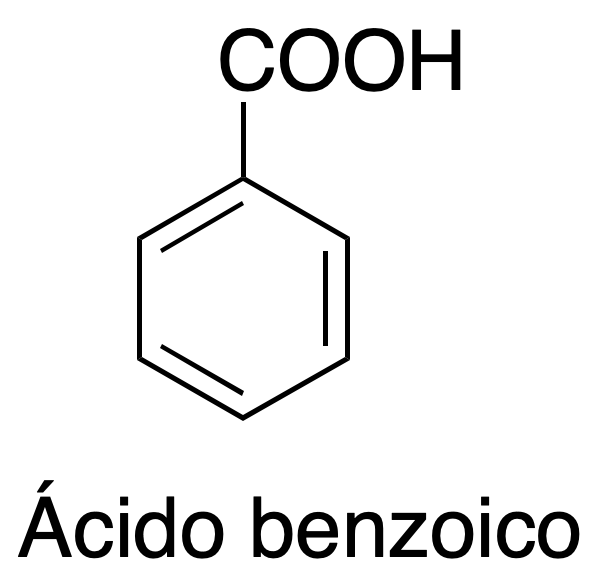
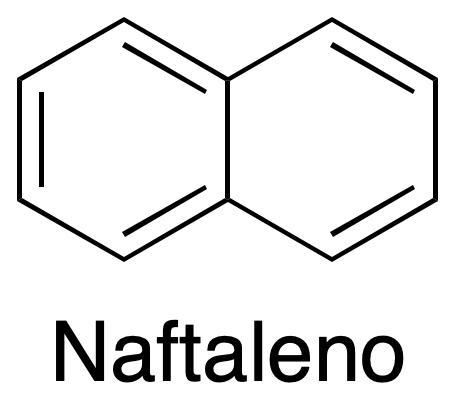
Liquid-liquid extraction is one of the most common basic operations in the organic chemistry laboratory, since many reactions involve the use of this technique for product isolation. Carboxylic acids can react with bases such as sodium hydroxide, producing a proton and forming the corresponding water-soluble anions (carboxylates). This experiment involves the separation of the components of a mixture consisting of naphthalene and benzoic acid, which will be dissolved in an organic solvent such as CH2Cl2, depending on the acidic or basic character of these compounds.
Procedure
Separation of two components (mixture of an acid and neutral compound): Take 30 ml of a problem solution containing an organic solvent (CH2Cl2) and two different dissolved compounds with neutral and acid character, such as: naphthalene, and benzoic acid, respectively.
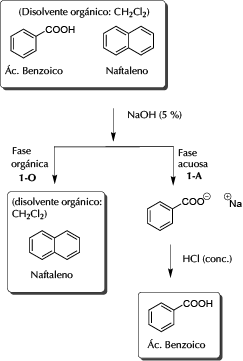
Place the 30 ml of the dissolution problem in a funnel of decanting of 250 ml. Add 40 ml of a 5% NaOH solution and shake vigorously (or with 20 ml twice). Let stand and then separate the two layers (the aqueous layer is the upper one and the organic layer, CH2Cl2, the lower one).
The organic layer 1-O (lower) is dried with anhydrous Na2SO4 for a few minutes. Remove the desiccant by gravity filtration. Transfer the filtrate to a dry round-bottomed flask, and evaporate the solvent to dryness at the rotary evaporator. The neutral component of the mixture is obtained as a solid of characteristic odor, naphthalene.
The aqueous layer 1-A is made acidic (use indicator paper) by adding small portions of HCl (conc.). Cool the mixture and vacuum filter (Kitasato and Büchner), the crystalline product obtained is the acid component of the problem mixture, benzoic acid.
General scheme of separation
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzoic acid | 122.12 | 125 | 249 | 1.08 |
| Naphthalene | 128.17 | 79.5-81.0 | 218 | - |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzoic acid |   |
| Naphthalene |    |
| CH2Cl2 |  |
| NaOH |  |
| HCl |   |
| Na2SO4 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzoic acid | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
| Naphthalene | UFWIBTONFRDIAS-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
Back to the Separation of Components of a Mixture experiment page.