Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To familiarize the student with the reactions that produce organic polymers, a polyamide such as nylon. In this case a very simple technique called “interfacial polymerization” is used.
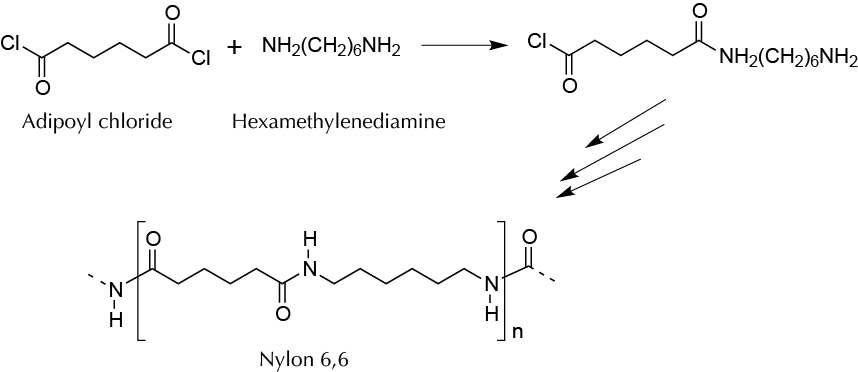
Background
Nylon is a synthetic polymer of the family of polyamides having great strength, providing the manufacture of fabrics for carpets, clothing, bands, ropes, hoses, conveyor belts, etc. It is synthesized by a polycondensation reaction of a diacid (or a derivative) with a diamine. Depending on the carbon atom number of the diacid and diamine, nylon is given the initials PA, the abbreviation for polyamide, and the number of carbons that the polymer gets from each of the two components. One of the most popular is PA 6,6 coming from adipic acid (hexanedioic acid) or its derivatives and hexamethylenediamine. In this experiment, PA polymer is prepared from hexamethylene diamine and adipoyl chloride in heterogeneous phase, forming the polymer at the interface.
Experimental procedure
In a 50-ml beaker, put 10 ml of aqueous hexamethylenediamine 5 %, and then add 10 drops of a solution of 20 % NaOH. Subsequently, add 10 ml of a solution (5 %) of adipoyl chloride in cyclohexane, pouring very carefully over the slightly inclined wall of the beaker. Two other organic and aqueous immiscible layers (cyclohexane) are formed, and immediately a polymer film appears at the in- terface. This technique is called “interfacial polymerization“.
With the help of a glass rod (or copper wire hook) gently peel the polymer from the walls of the beaker. The polymer is gathered in the center and is slowly raised using the glass rod, so that the polyamide formed continuously goes high and a large thread of nylon is produced. The thread may break if it stretches too quickly. Wash with water several times and let dry on filter paper. This operation is repeated as often as necessary. Finally, strongly agitate with the glass rod the two phases to produce greater amount of polymer. Let it dry for 24 h and weigh.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Adipoyl chloride | 183.03 | 105-107 | 112 | 1.259 |
| Cyclohexane | 84.16 | 4-7 | 80.7 | 0.779 |
| Hexamethylenediamine | 116.20 | 42-45 | 204-205 | 0.890 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Adipoyl chloride |  |
| Cyclohexane |     |
| Hexamethylenediamine |   |
| NaOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Adipoyl chloride | PWAXUOGZOSVGBO-UHFFFAOYSA-N |
| Cyclohexane | XDTMQSROBMDMFD-UHFFFAOYSA-N |
| Hexamethylenediamine | NAQMVNRVTILPCV-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- M. R. Dintzner, C. R. Kinzie, K. Pulkrabek, and A. F. Arena, The cyclohexanol cycle and synthesis of nylon 6,6: green chemistry in the undergraduate organic laboratory, Journal of Chemical Education 89 (2012), no. 2, 262–264, DOI: 10.1021/ed2000878