Written by J.A Dobado | Last Updated on April 22, 2024
Objective
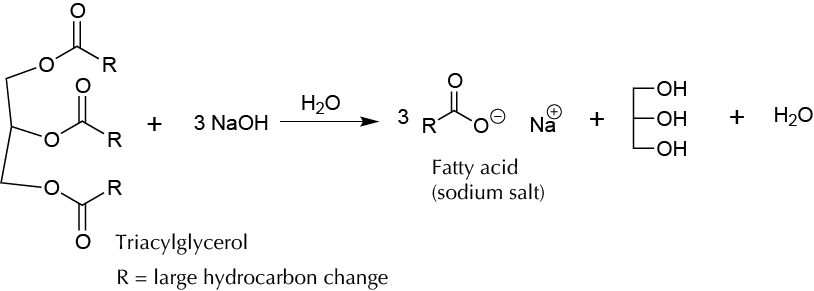
To make soap by reacting a vegetable oil with sodium hydroxide NaOH (saponification reaction).
Background
Soap (metallic or carboxylate ester) forms as a product of a saponification reaction. It is called the reaction between a carboxylic acid and a strong base such as sodium hydroxide NaOH or potassium hydroxide KOH. The main feature is the presence of soap in the molecule having two zones of different polarity: a hydrophilic (or lipophobic) and a lipophilic (or hydrophobic). The hydrophilic region located around the carboxyl group is strongly polarized and also forms hydrogen bonds with water molecules. The lipophilic region, which is very polar, is kept away from the water molecules and corresponds to the hydrocarbon chain.
Because of its dual amphiphilic character (hydrophilic-lipophilic), soap molecules have the property of solubilizing polar and non-polar molecules. Soap molecules show a strong tendency to migrate to the interfaces, so that the polar part is within the water and the apolar part is located facing an apolar part such as air or fat medium. This tendency to orient its structure with respect to water soap molecules lowers the surface tension at an air-water or oil-water interface, and therefore such molecules are called tensoactive agents.
Experimental procedure
In a 100 ml beaker, dissolve 9 g of NaOH in 12 to 15 ml of EtOH/water solution (50 %). In another 100 ml beaker, weigh 5 g of sunflower oil and add to the solution containing NaOH. Gently heat the mixture and stir continuously with a glass rod for 15 min. Remove the beaker from the hot plate if foam forms, and stir until the foam subsides. Cool the mixture and pour, while stirring, into a cold solution of 15 g NaCl in 60 ml of water. Afterward, cool to r.t., and then cool in an ice bath or place in the freezer. Cool the solution to precipitate the soap, which is isolated by vacuum filtration. Wash the resulting solid three times with cold water, dry, weigh, and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| NaCl | 58.44 | 801 | 1,413 | 2.165 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Vegetable oil |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| EtOH |  |
| NaCl | Non-hazardous |
| NaOH |  |
| Vegetable oil |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| NaCl | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Vegetable oil |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- J. A. Poce-Fatou, A superficial overview of detergency, Journal of Chemical Education 83 (2006), no. 8, 1147, DOI: 10.1021/ed083p1147
- C. S. Judd, News from online: cleaning up-soap, detergent, and more, Journal of Chemical Education 79 (2002), no. 10, 1179, DOI: 10.1021/ed079p1179