Written by J.A Dobado | Last Updated on April 22, 2024
Objective
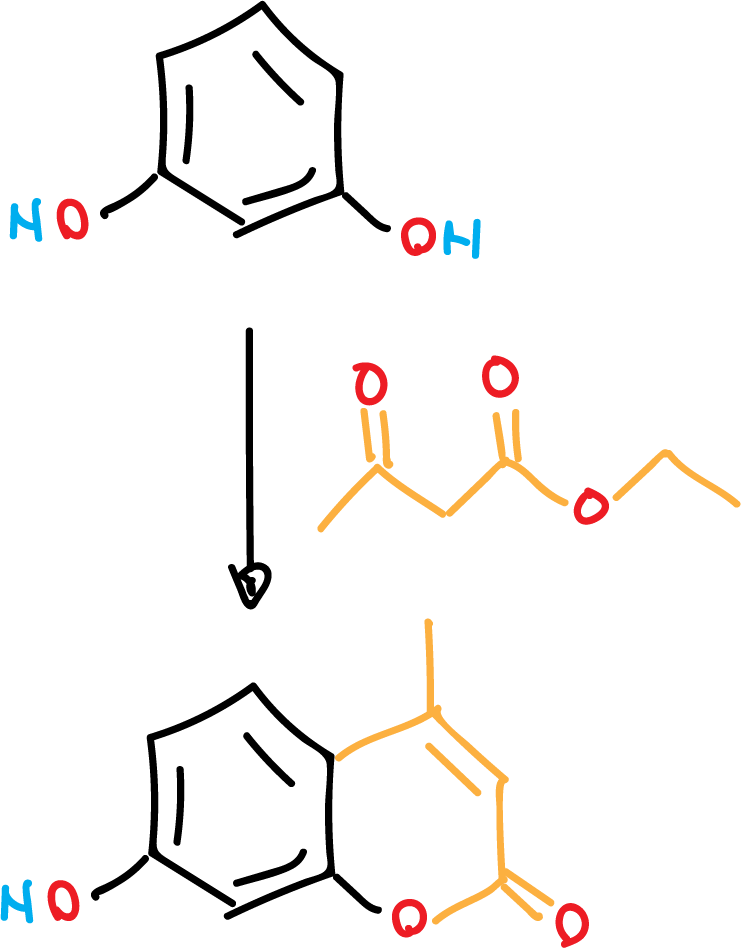
Synthesize a fluorescent compound 7-hydroxy-4-methyl-coumarin (7-hydroxy-4-methyl-2H-chromen-2-one) in a reaction carried out without solvent.
Background
Coumarins are natural products found in a wide variety of plants, including those used as traditional herbal medicines dating back to 1000 BC. Coumarins have been used for many practical applications, such as cosmetics, sunscreens, flavorings, laser dyes, pharmaceuticals and known anticoagulants. Coumarin 4-methylumbelliferone is used as a choleretic and antispasmodic drug and as a standard for fluorometric determination of enzyme activity, such as β-galactosidase. The target product is non-toxic, easy to prepare and isolate, and can be rapidly synthesized by Pechmann condensation by heating resorcinol and ethyl acetoacetate in the presence of a strong acid ion exchange resin (Dowex 50WX4). It also shows a pH-dependent fluorescence that is readily observed with a standard UV lamp.
Experimental procedure
In a 50 ml Erlenmeyer flask, place ethyl acetoacetate (1.0 ml, 1.02 g, 7.8 mmol), resorcinol (800 mg, 7.3 mmol) and Dowex 50WX4 beads (1.0 g). Place the flask on a hotplate set to the lowest heating level.
The reaction proceeds at a reasonable rate at temperatures as low as 80 °C, and the onset of the reaction is indicated by gentle bubbling. Stir the mixture occasionally or stir with a glass rod until the bubbling stops and the mixture solidifies to a tan-colored solid (usually 20 to 30 min).
Add a small volume (2-3 ml) of hot 95 % EtOH to dissolve the solid and then use a Pasteur pipette to transfer the hot solution from the Dowex beads to a clean Erlenmeyer flask. If desired, the beads can be rinsed with an additional portion of hot (95%) EtOH.
While the mixture is heating, add hot water until the solution becomes slightly cloudy, remove the flask from the hot plate and allow to cool slowly to room temperature.
Isolate the white to off-white crystalline precipitate by vacuum filtration and wash with water. Allow the product to dry before determining the melting point (179-183 ºC; estimated yields from 50 to 65 %)
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Ethyl acetoacetate | 130.14 | -43 | 181 | 1.029 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Resorcinol | 110.11 | 178 | 109-112 | 1.272 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Ethyl acetoacetate |  |
| EtOH |  |
| Resorcinol |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Ethyl acetoacetate | XYIBRDXRRQCHLP-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Resorcinol | GHMLBKRAJCXXBS-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. M. Young, J. J. C. Welker, and K. M. Doxsee, Green Synthesis of a Fluorescent Natural Product, Journal of Chemical Education 88 (2011), no. 3, 319–321, DOI: 10.1021/ed1004883