Written by J.A Dobado | Last Updated on April 22, 2024
What is the structure of methyl orange?
(C14H14N3NaO3S) Methyl orange is an organic compound with a chemical structure characterized by a central aromatic benzene ring that contains a nitrogen atom. This nitrogen atom is bonded to two methyl groups (-CH3) and an oxygen atom (-O) within the ring.
What is methyl orange?
In 1865, William Perkin revolutionized the food, textile, cosmetics, paper, leather and paint industries by preparing the first synthetic organic dyes. Methyl orange (helianthin, Poivrier orange, gold orange or orange III) of molecular formula (C14H14N3SO3Na) is a sodium salt of a diazocompound. The IUPAC systematic name is sodium 4-{[4-(dimethylamino)phenyl]diazenyl}benzene-1-sulfonate.
Structure
The main structural characteristic of methyl orange is that it has an azo group (-N=N-) attached to two substituted aromatic rings, this is a characteristic of azo dyes, and represents the class of compounds most commonly used in textile and food processing.
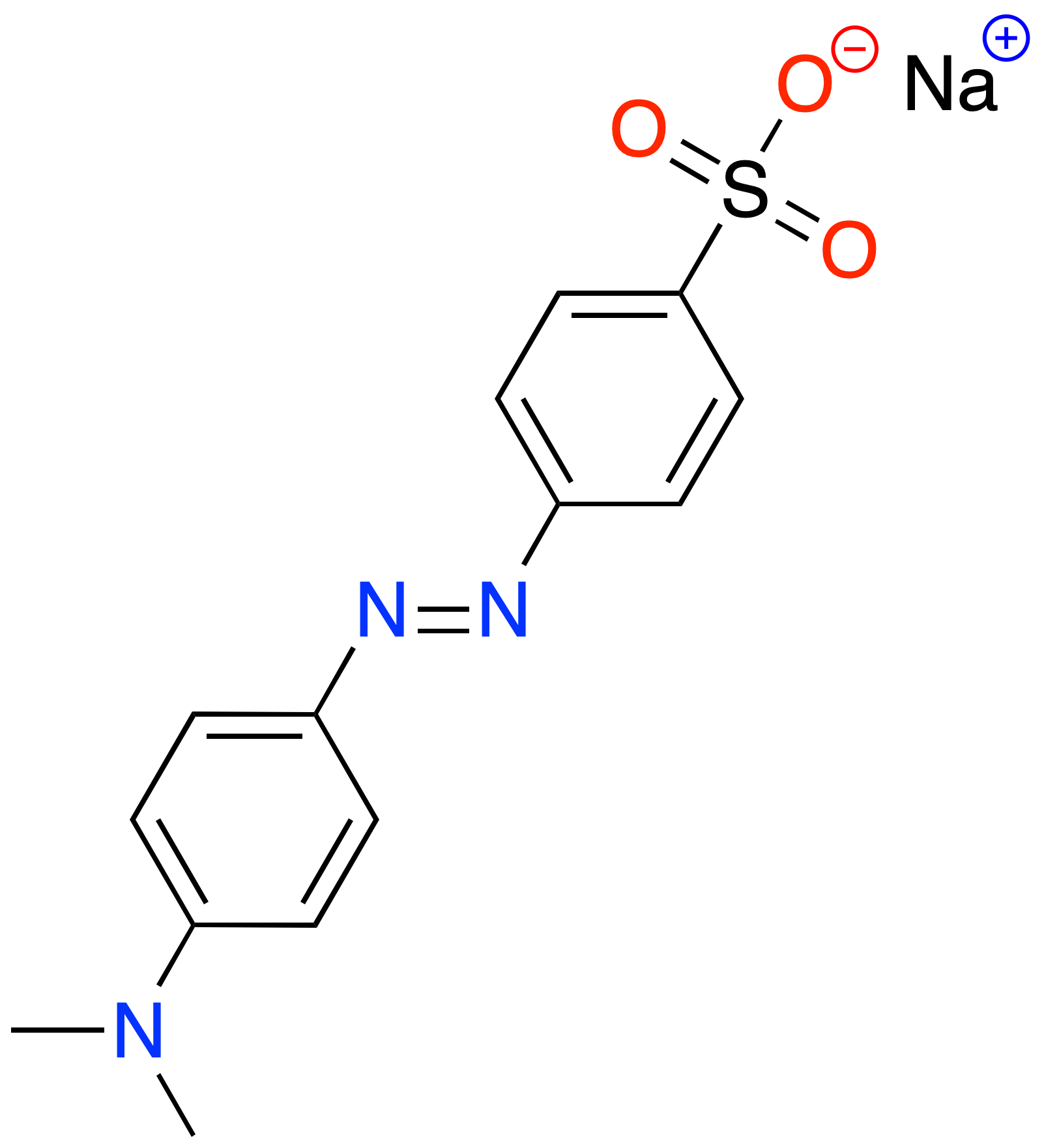
3D Structure
Physico-chemical properties
The ionic state of methyl orange makes this compound essentially non-volatile. It is an orange-red solid that has no odor (odorless) and exhibits the following physicochemical properties: molecular mass is 327.34 g/mol, boiling point > 300 °C, density 1.28 g/cm3, being soluble in 500 parts water and insoluble in alcohol.
Methyl orange can exist mainly in two different forms, the alkaline (deprotonated) and the acidic (protonated) form. For pH above 5.5, there is only an anionic form.
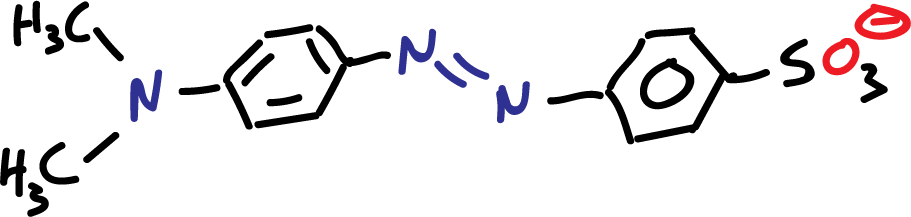
At lower pH values (acidic), a fraction of particles changes and for very low pH (pH <2.0) is only present in three species of the protonated form which present two opposite charges as indicated in the figure:
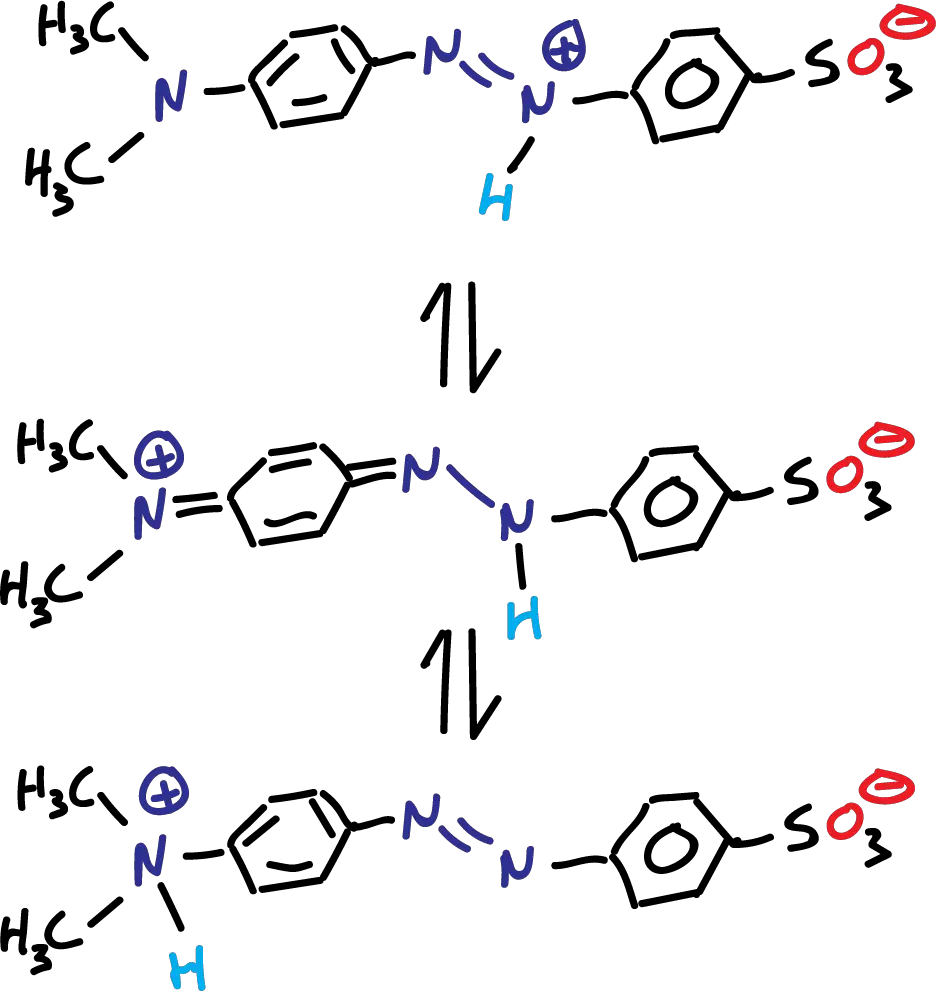
Three species formed in the protonation (H⊕) of methyl orange
When methyl orange is brought to acidic pH, the H⊕ binds to an azocompound nitrogen to form an azonium ion. Positive charge is then distributed to the nitrogen at the end of the molecule.
Although the dimethylamino group has a lower affinity for H⊕, ammonium ion formation can also occur at low pH. A tautomeric equilibrium is established between the ammonium and azonium ions.
The color change that methyl orange undergoes at different pH is due to the different species that are formed. As well as in the way electrons are regrouped molecule when a proton is attached or detached (H⊕).
In the pH zone (>4.4) weak acidic, neutral and basic, its coloration is yellow-orange, and for pH (<3.1) strongly acidic it changes to red.
Uses
In 1878, it began to be used as a chemical indicator of pH, it is used at a concentration of 1 drop at 0.1 % per 10 ml of solution. Its range is between 3.1-4.4, and its color is red below 3.1 and orange-yellow above 4.4.
Today it is widely used in the chemical and pharmaceutical industry, etc. and as a dye in the textile industry, as well as a dye in cytology (together with Fuschin’s solution).
GHS classification
Pictogram: Toxic

Health hazard H-phrase: H301
P-phrase for caution: P264, P270, P301+P316, P321, P330, P405, P501
Reactivity profile
Methyl orange is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Video on methyl orange indicator
International Chemical Identifier
We provide identifiers InChI key of the IUPAC for, the main compounds described in this section in order to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| methyl orange | STZCRXQWRGQSJD-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145
- Phenolphthalein and methyl orange
Charles A. Peters and Bryan C. Redmon
Journal of Chemical Education 1940 17 (11), 525
DOI: 10.1021/ed017p525