Written by J.A Dobado | Last Updated on April 22, 2024
What is hydrogen sulfide?
Hydrogen sulfide is an inorganic compound with the formula H2S composed of one sulfur and two hydrogen atoms. It is the analog of water but exchanges oxygen for sulfur.
H2S is a gas with a characteristic odor of rotten eggs, and is present in nature in many volcanic fumes. However, this molecule has essential functions in the human organism. It is found in petroleum, natural gas, or gases emitted in volcanoes and hot springs.
Chemical structure
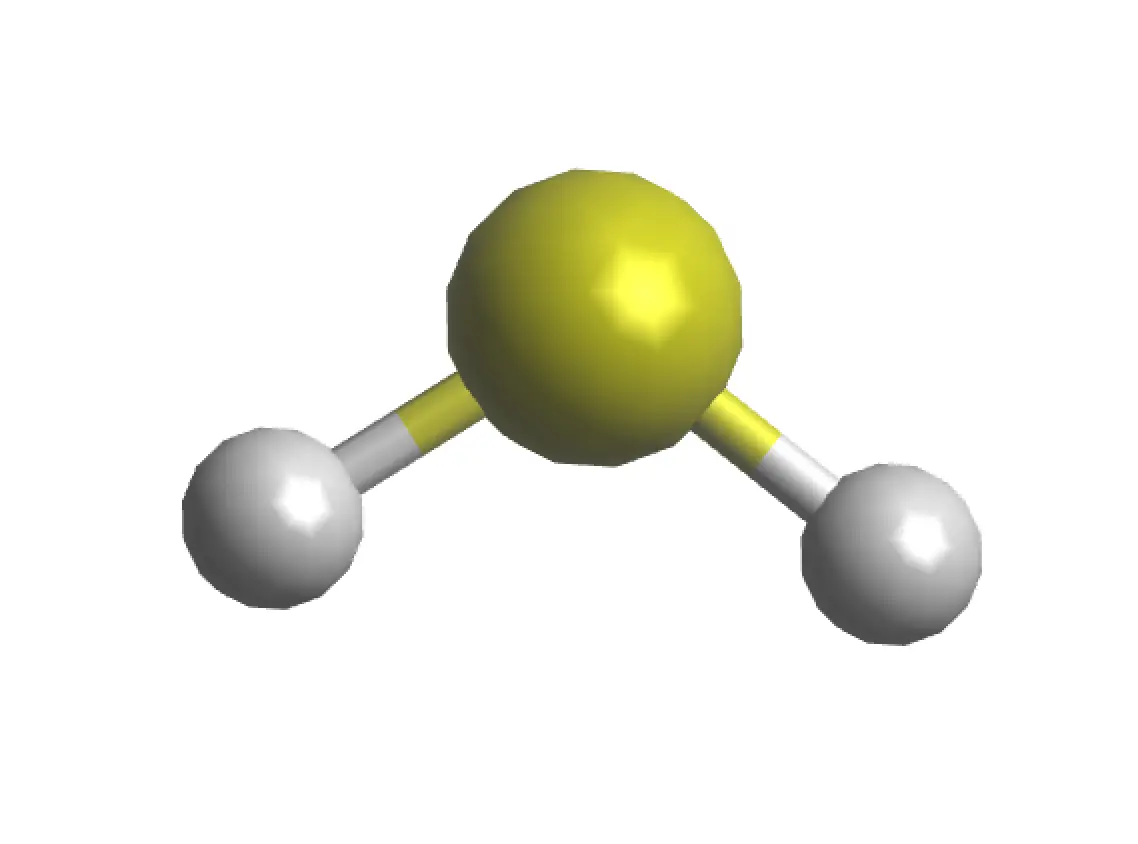 |
| 3D Structure |
The hydrogen sulfide molecule presents an angular geometry (similar to water), with an S-H bond length of 1.336 Å and a bond angle ∠HSH of 92.1º. Regarding its molecular symmetry, it belongs to the C2v point group. In addition, it has a dipole moment of 0.97 Debye.
It can be represented by the following Lewis structure (see step by step explanation):
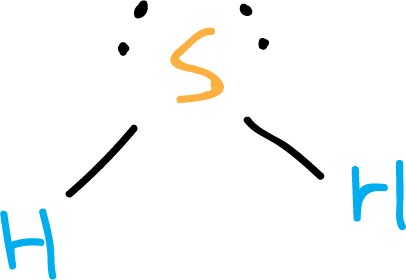
Physico-chemical properties
It is a heavier gas than air. In addition, it is flammable, colorless, toxic, odoriferous (decomposing organic matter).
Hydrogen sulfide when in aqueous solution is called (H2S(aq)), and has a solubility in water of 4 g dm−3 (at 20 °C), and it is also soluble in alcohol. H2S has a molar mass of 34.08 g/mol. It is basic in nature (pKa of 6.89), so in the presence of strong bases it forms salts called sulfides.
It has a boiling point of -60.29 ºC and a melting point of -86 ºC and density 1.363 g dm−3. It burns with a blue flame in oxygen to form sulfur (IV) oxide and water.
H2S is very harmful to health. Concentrations of only 20-50 parts per million (ppm) in the air cause acute discomfort leading to asphyxiation and death from overexposure.
Its toxicity is comparable to that of hydrocyanic acid (HCN). The latter was used in gas chambers and concentration camps during World War II.
The presence of H2S in a sample can be detected by a test impregnating a wet paper with lead acetate, Pb(CH3COO)2, lead nitrate can also be used. A positive result will result in a black spot due to lead sulfide formed by the following reaction:
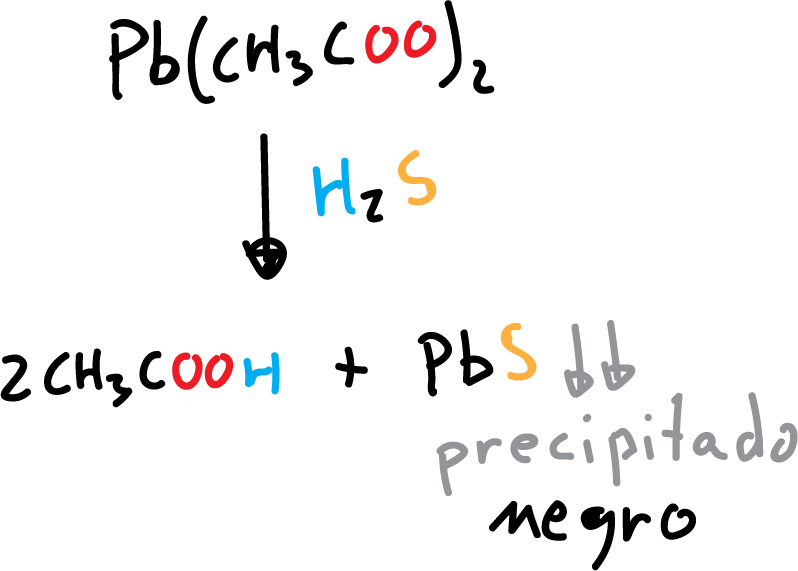
Obtaining
It can be obtained by decomposition of organic matter under anaerobic conditions. H2S originates from proteins with sulfur amino acids (cystine, cysteine and methionine). These sulfur amino acids are more abundant in hair, nails and horns.
Another method, obtained by the action of anaerobic bacteria, is achieved by the reduction of sulfates (SO4=) present in water, by contact of a sulfide or sulfhydrate with an acid, with acidic or even neutral water, or by combustion of a hydrocarbon or hydrogen in contact with sulfur.
In the laboratory, it is obtained by reaction of hydrochloric acid (HCl) with iron(II) sulfide (FeS), using a kipp generator (Kipp’s apparatus).
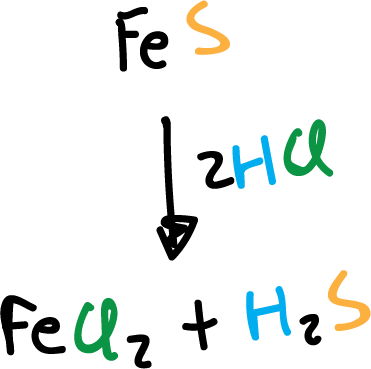
Also, thioacetamide is used as a starting product to obtain it for use in Qualitative Inorganic Analysis.
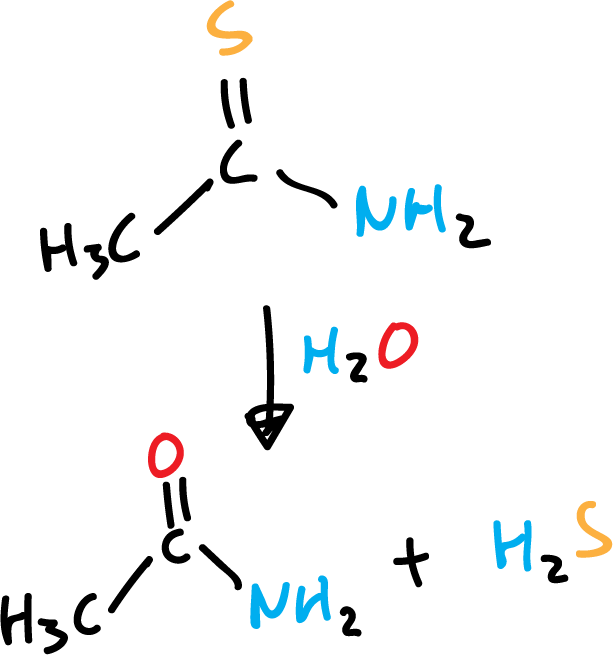
Many metal and non-metal sulfides (aluminum sulfide, phosphorus pentasulfide, silicon disulfide) release hydrogen sulfide when mixed with water.
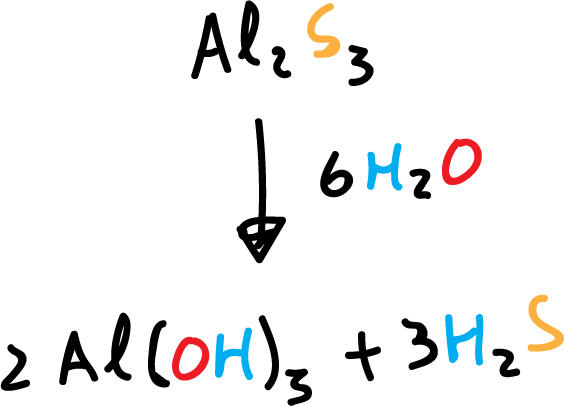
Another laboratory method consists of heating a mixture of elemental sulfur (S) and kerosene.
On an industrial scale, hydrogen sulfide is obtained as a by-product of natural gas cleaning, in which it is found in concentrations of up to 10%.
Uses and applications
Formerly used in qualitative analytical chemistry, in the cationic march (analytical march) to precipitate heavy metal cations of group II. Mostly black or white precipitates are obtained.
Sodium sulfide salt (Na2S) is used to age (antique simulation) bronzes (goldsmiths). It is also used in tanneries for the preparation of leather.
Hydrogen sulfide is responsible for the blackening of some lead carbonate-based paints by the formation of plumbosulfide (lead sulfide II), (PbS), which is black in color.
Hydrogen sulfide is a starting compound of some Organic Syntheses.