Written by J.A Dobado | Last Updated on April 22, 2024
What is colchicine?
La colchicine it is an alkaloid that is found in the wild saffron (Colchicum autumnale). Its use in medicine are described for the first time in the Ebers papyrus (medical papyrus Egyptian approximately 3500 years old), as a remedy anti-inflammatory and anti-arthritis (gout attacks).
Colchicine is a solid in the form of needles or powder pale yellow that darkens with exposure to light. It is odorless or almost odorless. Molecular formula C22H25NO6 and molecular mass of 399.4 g/mol. Its melting point is 142-150 °C.
The solubility in water at room temperature (25 ºC) is 4.5 g in 100 ml of water. In addition, 1 g of colchicine dissolved in 220 ml of ether, in 100 ml benzene. It is very soluble in alcohol or chloroform, and practically insoluble in petroleum ether (hexane).
Structure
Features three rings fused (2 of 7 members, and one of 6), with a chiral center at the group acetamide in one of the rings heptagonales of tropona. In addition, as substituents additional features 4 groups, methoxyl (CH3O–), 3 at the benzofused ring and 1 at the tropolone one. The IUPAC systematic name is the N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide.
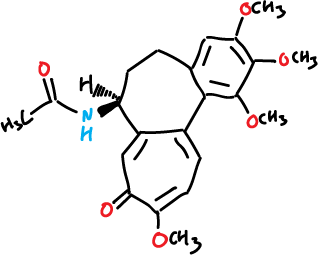 |
| 3D Structure |
Although it presents only one chiral carbon at the C7 position, due to the relative orientation of the rings, 4 different stereoisomers are possible (depicted in the figures), being obtained naturally in the wild saffron corresponds to the (−)-(aS, 7S)-colchicine isomer. The molecule presents atropoisomery been assigned to this asymmetry two labels aS or aR, where a designates an axial chirality for this type of esteroisómeros. The C—C bond between the ring (benzene ring) and the C-ring (unsaturated 7-membered one) acts of chirality axis.
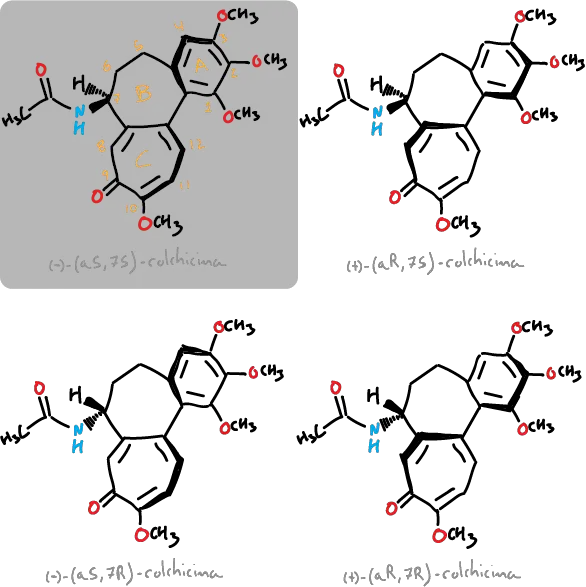
In addition, the energy barrier of interconversion is approximately 22-24 kcal·mol-1 among the forms aR and aS, with the latter of the alkaloide that is found in nature.
Biosynthesis
Biosynthesis in plants, colchicine is carried out with amino acids tyrosine and phenylalanine as precursors.
It has been described that the tropolone ring of colchicine is formed from the expansion of the tyrosine ring. It is derived biosynthetically from (S)-autumnaline. This biosynthetic pathway occurs by means of a coupling reaction phenolic that leads to the intermediate isoandrocymbine.
Subsequently, this intermediate undergoes O-methylation directed by S-adenosylmethionine. Biosynthesis continues with two oxidation steps followed by the cleavage of the cyclopropane ring leading to the formation of the tropolone ring contained by N-formyldemecolcine.
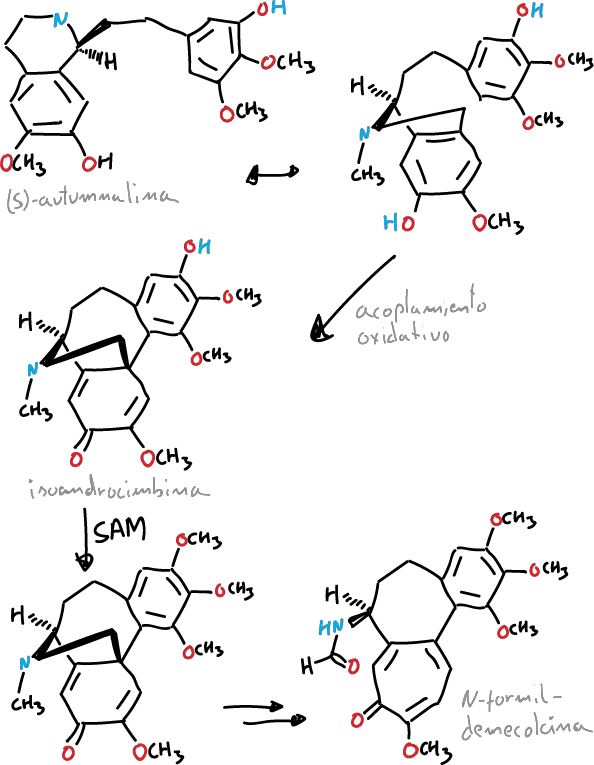
La colchicine can be purified from the wild saffron (Colchicum autumnale), finding himself in a maximum concentration in summer vary from 0.1% in the flower to 0.8% in the seed and the bulb.
However, it is not available in sufficient quantities for commercial use. Gloriosa superba it is another plant that also contains colchicine, and is obtained, alternatively, from its dry tubers.