Written by J.A Dobado | Last Updated on April 22, 2024
What is limonene?
Limonene is a natural product extracted from the peel oil of citrus fruits (lemon, orange, etc.). Its molecular formula is C10H16 and molecular mass is 136,238 g·mol−1. It is a colorless or pale yellow liquid. Its IUPAC name is 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene. It has the characteristic odor of oranges and lemons. It is an organic compound of the terpene family.
Applications
Its main application today is as a biodegradable industrial solvent. It is also used in organic synthesis to obtain other chemical compounds. In the food industry (flavoring), pharmaceuticals, cosmetics and also as a solvent for resins, inks, pigments, manufacture of adhesives, etc.
Chemical structure
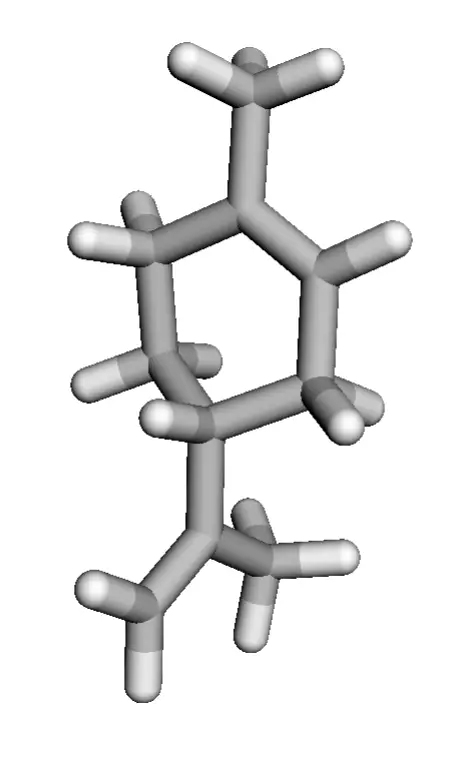
Limonene is a chiral compound and therefore has two enantiomers. Consequently, there are two optical isomers: the enantiomer R-limonene and S-limonene. They are also called D-limonene and L-limonene (formerly called dextro and levo, respectively). Dextrorotatory (+) limonene is an oily liquid that can be easily extracted from lemon peel and imparts the characteristic lemon odor. Levorotatory (-) limonene, however, is extracted from orange peel and has an orange odor.
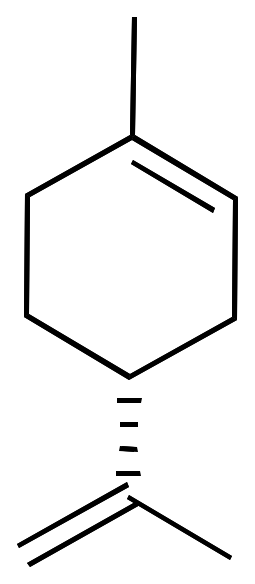 | 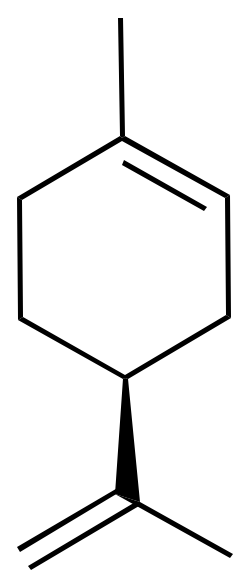 |
| 3D Structure | 3D Structure |
Physico-chemical properties
R-Limonene is obtained commercially from lemon and has a chiral rotation, [α]D, of 87-102°. Limonene is a relatively stable monoterpene and can be distilled without decomposition, although at elevated temperatures it fragments to form isoprene.
It has a density of 0.8411 g· mol–1, with a melting point of -74.35 ºC and a boiling point of 176 ºC. Insoluble in water and miscible with other organic solvents such as: strong>benzene, chloroform, ether, CS2 and CCl4 It has a refractive index nD = 1.4727.
Method of production
Biosynthesis
In nature, limonene is formed from geranyl pyrophosphate by cyclization of a neryl carbocation or its equivalent. The final step involves the loss of a proton from the cation to form the alkene.
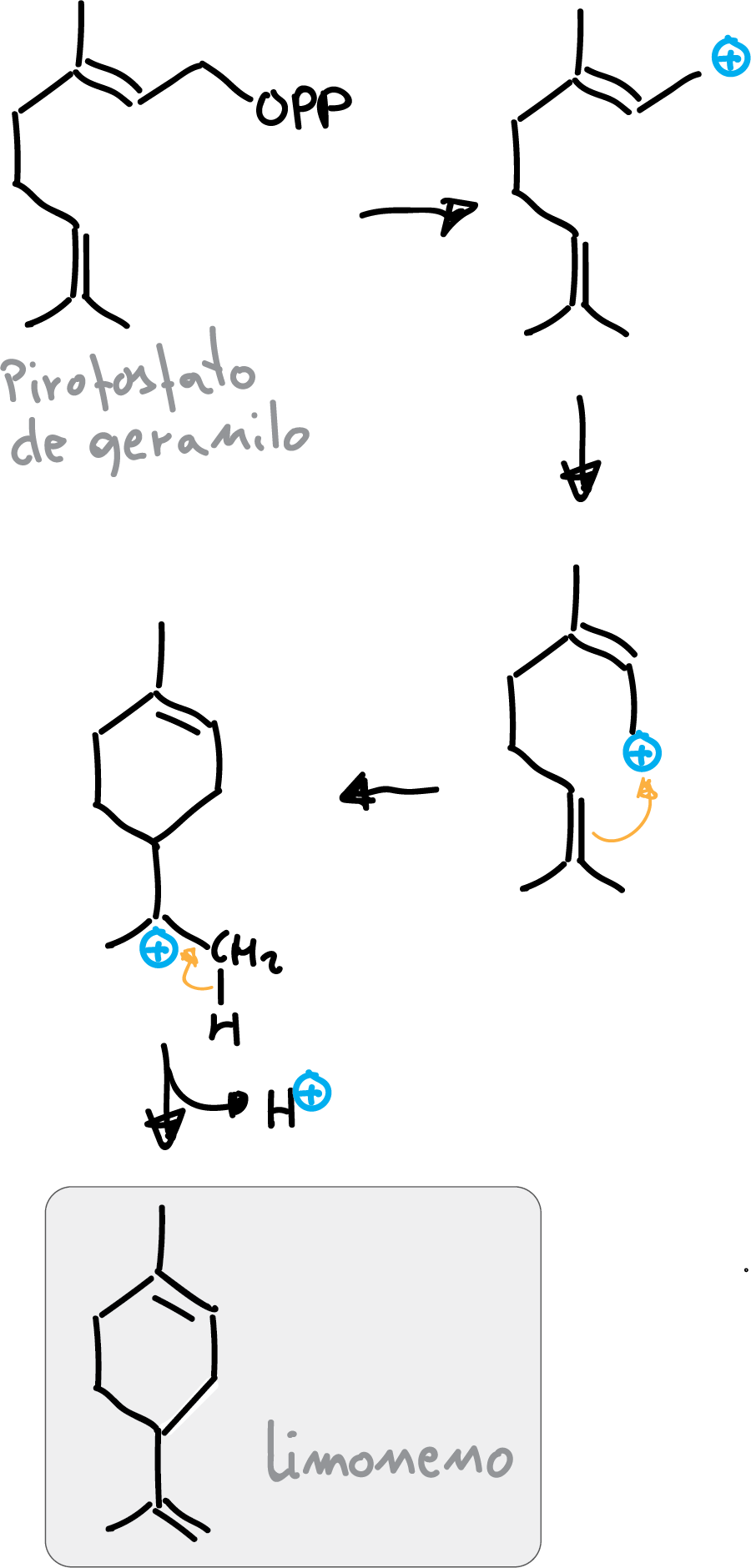
Limonene reactions
In Organic Synthesis, limonene is used as starting material to obtain carvone. Carvone is a high value-added compound used in the pharmaceutical and food industries (with anti-microbial and anti-fungal properties).
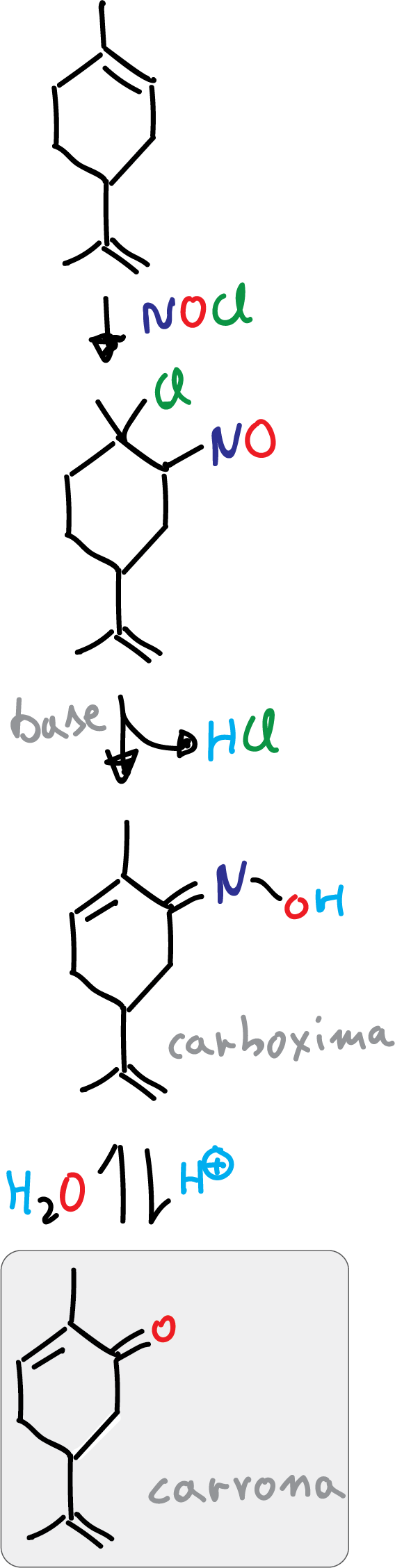
The synthesis consists of three steps, starting with the regioselective addition of nitrosyl chloride to the trisubstituted double bond. Subsequently, this intermediate is converted to the oxime with a base. Finally, the hydroxylamine obtained is removed to give rise to the carvone which exhibits a ketone.
On the other hand, limonene is readily oxidized in humid air to produce carveol, carvone and limonene oxide. With sulfur, it dehydrogenates to p-cymene.
Limonene commonly occurs as the R enantiomer, but racemizes to dipentene at 300 ºC. When heated with mineral acid, limonene isomerizes to the conjugated diene α-terpinene (which can also be readily converted to p-cymene).
It is possible to perform the reaction on one of the double bonds selectively. The Anhydrous HCl reacts preferentially on the disubstituted alkene, whereas the epoxidation with mCPBA occurs on the trisubstituted alkene.
In another synthetic method, Markovnikov addition of trifluoroacetic acid to limonene followed by hydrolysis of the acetate results in the compound terpineol.
GHS classification
Pictogram: hazard



H-phrase physical hazard:
- H226
H-phrase health hazard:
- H304, H315, H317