Written by J.A Dobado | Last Updated on April 22, 2024
What is ozone?
Ozone, with the general formula O3, is a molecule composed of three oxygen atoms. It is a pale blue gas, which upon cooling to low temperatures condenses into a liquid, with a boiling point of -192.2 ºC, dark blue in color, and finally solidifies with a violet-black color and a melting point of -112 ºC.
It has a pungent odor, reminiscent of chlorine. Being more unstable than oxygen gas, O2, it decomposes easily. This means that, industrially instead of storing and transporting it, when it is needed, it is generated in situ. In addition, as it oxidizes metals, oxidation resistant materials such as titanium, glass, stainless steel, aluminum or polymers such as Teflon (polytetrafluoroethylene, PTFE) must be used. Due to its instability it is used in industry in low concentrations.
It is found at very low concentrations in the lower parts of the atmosphere, and at high concentrations in the stratosphere. There, it absorbs most of the sun’s ultraviolet UV radiation.
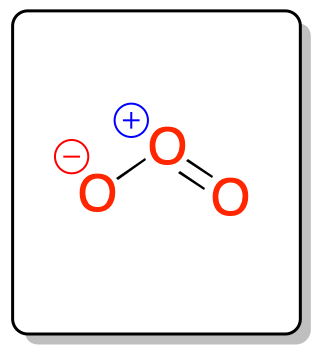
Structure
La estructura del ozono, O3, se determinó por primera vez en 1865. Subsequently, it was shown to have an angular structure, unlike, for example, carbon dioxide (CO2), which, although it consists of three atoms, has a linear structure.
Generation
In nature, ozone is generated in the upper atmosphere due to ultraviolet radiation, and can also originate in association with electrical discharges from lightning.
Artificially, ozone can be generated in two ways that simulate these two natural phenomena: by silent corona discharge, and by ultraviolet (UV) radiation.
Ultraviolet radiation
Ultraviolet (UV) generation is like the process of creating ozone in the upper atmosphere. It therefore involves the use of a mercury lamp (λ < 240 nm) and creates problems around material waste.
O2 —(fotón)→ 2O
The ozone molecule thus formed can now absorb a photon of UV energy and dissociate into oxygen.
O3→ O2 + O + energía cinética
Corona discharge
The crown effect is a phenomenon produced by the ionization of the gas surrounding a charged electrical conductor. It is observed spontaneously in high voltage lines, and is seen in the form of a luminous halo. Thus, because the wires have a circular cross-section, the halo is shaped like a crown, hence the name. This method is similar to how ozone is generated in lightning. The corona diccharge method produces more ozone than the UV method and is also a much more efficient process.
In the corona discharge method, ozone is generated by means of an electric discharge from oxygen that has been previously cleaned.
The oxygen is cleaned in dryers and filters. Subsequently, it undergoes a homolytic breakage into two oxygen atoms by means of the electrical discharge.
O2 → 2O
Subsequently, they react with an O2 molecule to generate ozone.
O2 + O + M → O3 + M
M intervenes, which is a third component that absorbs the excess energy of the reaction.
Effects of ozone on health
Ozone can damage the lungs, causing chest pain, coughing, shortness of breath and throat irritation. It can also worsen chronic respiratory diseases such as asthma and compromise the body’s ability to fight respiratory infections, even in healthy people. People with asthma and allergies are more prone to the adverse effects of high ozone levels. For example, increasing ozone concentrations to unsafe levels can increase the risk of asthma attacks.
The effects of acute and chronic exposure to ozone on human health have been studied. Thus, many studies suggest that ozone is harmful to people at levels currently found in urban areas (see Stewart y cols. and US EPA report). Ozone has also been shown to affect the respiratory, cardiovascular and central nervous systems. In addition, ozone exposure is associated with early death and reproductive health and development problems.