Written by J.A Dobado | Last Updated on April 22, 2024
What is uric acid?
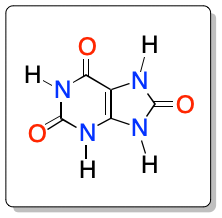
Uric acid is an organic molecule derived from xanthine with the chemical formula C5H4N4O3. It has the IUPAC systematic name 7,9-dihydro-3H-purine-2,6,8-trione.
This heterocyclic nitrogenous compound is an end product of the catabolism of purines (adenine and guanine) that takes place through the action of the enzyme xanthine oxidase (from xanthine and hypoxanthine). Uric acid is also the end product of nitrogen metabolism in birds and reptiles. In such species, it is excreted in the feces as a dry mass.
 |
| 3D Structure |
Physico-chemical properties
This purine derivative is a white crystalline solid with a melting point of 300 ºC and a molar mass of 168.1103 g·mol-1. It has a low solubility in water and ethanol, characteristic of this type of nitrogenous heterocycles, and relevant in gout problems. High levels of uric acid in the blood (hyperuricemia) lead to gout disease. Due to the accumulation of monosodium crystals of this acid in the joints, soft tissues and joints.
However, their alkali and alkaline earth metal salts are more soluble in hot water, which allows them to easily recrystallize.
On the other hand, this heterocycle presents a keto-enol tautomerism (lactam-lactide):
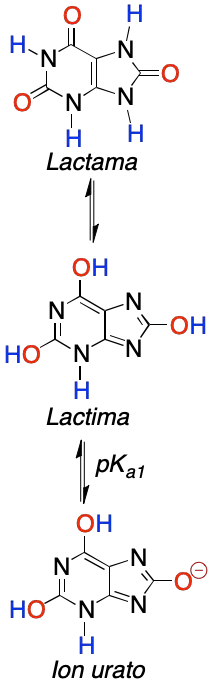
However, the form expected to be the most stable would correspond to the lactide (more aromatic). However, the one obtained experimentally is the lactam, because it crystallizes in that form.
Furthermore, being a diprotic acid, it has two pKa (pKa1 = 5.4 and pKa2 = 10.3) values. At physiological pH, the major structure is the monoionic urate ion.
Synthesis
Uric acid was first obtained in 1776, by Carl Wilhelm Scheele, from kidney stones.
Later, in 1882, Ivan Horbaczewski synthesized it for the first time in the laboratory by fusing urea and glycine.