Written by J.A Dobado | Last Updated on April 22, 2024
What is sulfuric acid?
It is a mineral acid with the general formula H2SO4. In ancient times it was referred to as vitriol, vitriol oil, vitriolic acid and vitriol spirit.
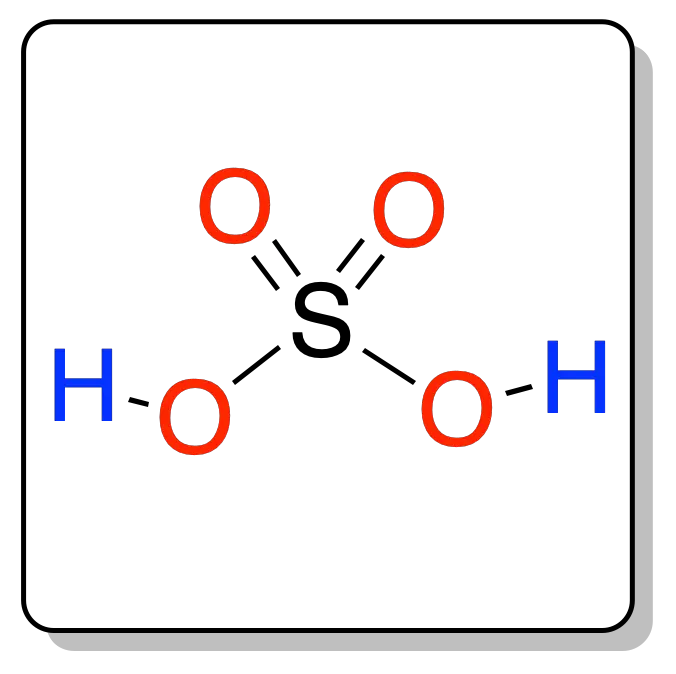 |
| 3D Structure |
Physico-chemical properties
It is a viscous, colorless, odorless liquid, soluble in water. It has a molecular mass of 98.079 g·mol-1. It has a density at 15 ºC of 1.8342 g·ml-1, a melting point of 10.5 ºC, and a boiling point of 274 ºC.
It has a strong acidic character that makes it very corrosive in high concentrations. It also has dehydrating and oxidizing properties. In addition, it is hygroscopic so it easily absorbs water vapor from the air.
| DANGER! “it is corrosive and causes severe chemical burns, even at even lower concentrations.” |
When we must dilute it with water, it is necessary to do it pouring the acid on the water, slowly and shaking.
Acid-base properties
Because of its acidic character, H2SO4 reacts with most bases to give the corresponding sulfate.
For example, cupric sulfate or copper (II) sulfate, which is used as a phytosanitary product, is prepared by the reaction of copper (II) oxide with sulfuric acid:
CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l)
In addition, being a strong mineral acid, it has the ability to displace other weaker acids. For example, it displaces the salt of acetic acid (sodium acetate) when it reacts as follows to form sodium bisulfate:
H2SO4 + CH3COONa → NaHSO4 + CH3COOH
Similarly, the reaction of sulfuric acid with potassium nitrate produces nitric acid and a precipitate of potassium bisulfate.
In addition, and of particular utility in organic electrophilic aromatic substitution reactions (SEAr) is the reaction that takes place with nitric acid to give the nitronium ion (NO2⊕).
Production
In industry, sulfuric acid is a notable chemical, and countries’ production data are used as an indicator of their industrial potential.
Therefore, it is an essential product in the chemical industry. It is widely used in the manufacture of fertilizers, explosives, mineral processing, wastewater treatment, petroleum refining, etc.
In addition, some of the most relevant end-use applications are: domestic drain uncloggers, electrolytes in lead batteries, cleaning agents, etc.
Although sulphuric acid was discovered in the 8th century, its production only became economically viable in 1746, when chemist John Roebuck developed a way to produce it in bulk.
Nowadays, different H2SO4 production methods are used: such as the contact process, the wet sulfuric acid process, the lead chamber process, etc.
Contact method
In the first stage of the process, sulfur is burned to obtain sulfur dioxide:
S (s) + O2 (g) → SO2 (g)
The sulfur dioxide is then oxidized to sulfur trioxide using oxygen and a vanadium catalyst. The reaction is exothermic and reversible.
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g) (in the presence of the V2O5 catalyst)
The sulfur trioxide is then absorbed in 97-98 % sulfuric acid to form disulfuric acid (H2S2O7), also known as fuming sulfuric acid. It is then diluted with water to obtain concentrated sulfuric acid.
H2SO4 (l) + SO3 (g)→ H2S2O7 (l)
H2S2O7 (l) + H2O (l) → 2 H2SO4 (l)
Directly dissolving SO3 in water is not practical, due to the very exothermic nature of the reaction. In addition, this reaction forms a corrosive aerosol, rather than a liquid, which is very difficult to separate.
SO3 (g) + H2O (l) → H2SO4 (l)