Written by J.A Dobado | Last Updated on April 22, 2024
What is the electrophilic aromatic substitution reaction (SEAr)?
These are characteristic reactions of the aromatic ring of benzene and derivatives, involving the substitution of one hydrogen of the ring by another group that has an electrophilic character (E⊕), is positively charged or is a reactant capable of forming a bond with two electrons of the π cloud of benzene.
The benzene ring acts as a source of electrons (base) against the electron-deficient (acidic) E⊕ electrophiles. (see also SEAr named organic reactions)
Examples of SEAr
SEAr reactions include a very wide variety of reactions. Examples of these reactions are: sulfonations, nitrations, halogenations, Friedel-Crafts reactions. The latter can be used on almost all aromatic rings.
In addition, other reactions such as nitrosation or diazo coupling, also occur but the rings of higher reactivity, and also include desulfonation reactions, isotopic exchange and many ring closures.
Reaction mechanism
As benzene presents double bonds, they act as nucleophiles, reacting with different types of positively charged electrophiles E⊕.
The first step of the reaction takes place with the attack of a pair of electrons from the benzene to the electrophile forming a C-E bond, and placing the charge on the adjacent carbon. However, this charge is delocalized by resonance between the three resonant structures of the cyclohexadienyl cation shown in the scheme. This carbocationic intermediate is called Wheland intermediate or σ-complex.
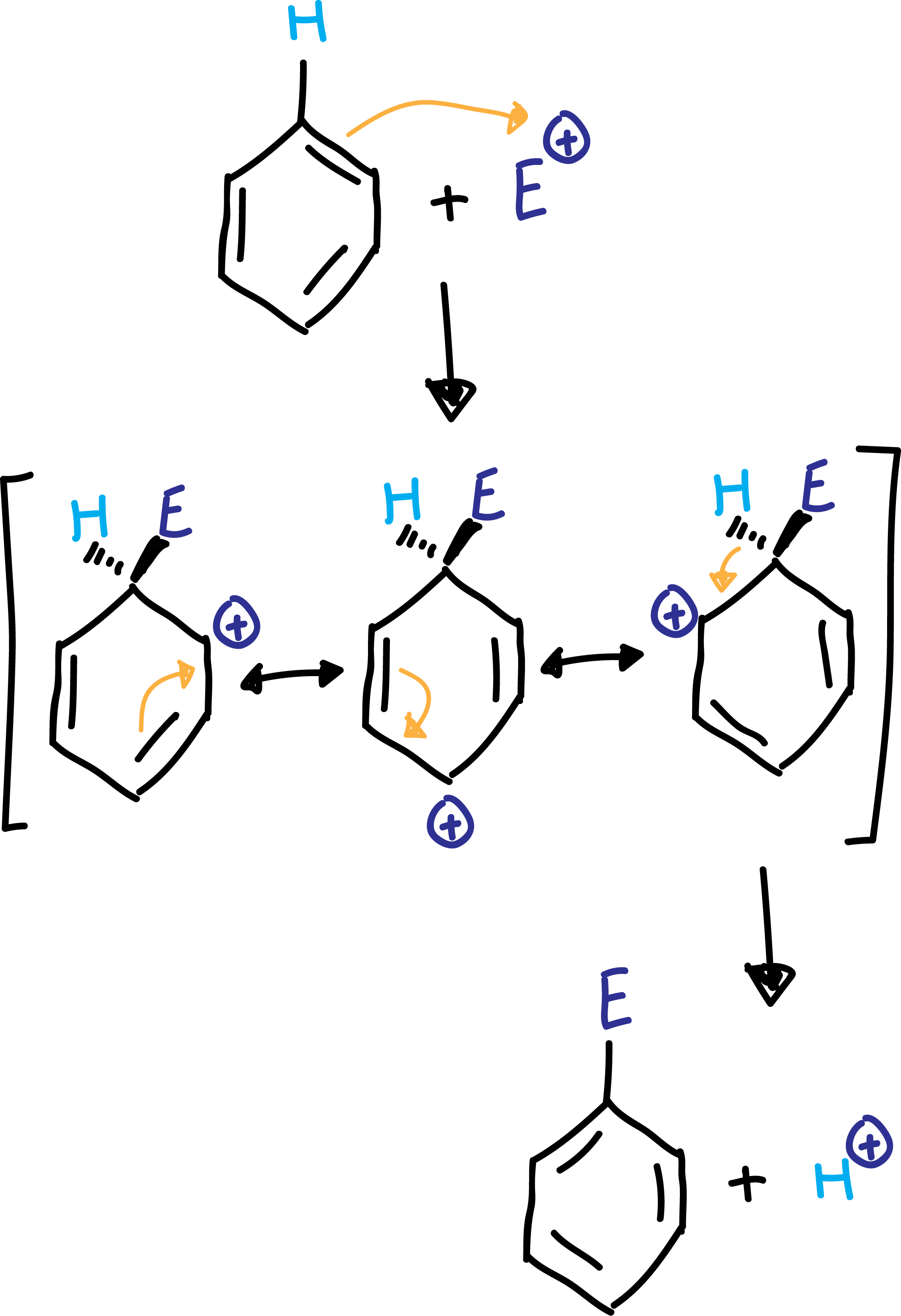
Finally, in a second step, a proton is lost, so that the benzene regains its aromaticity (rearomatization of the ring).