Written by J.A Dobado | Last Updated on April 22, 2024
Nomenclature of linear ethers
They have the R-O-R’ or Ar-O-Ar’ functional group (recommendation C-211.1). There are two types of nomenclature and depending on the complexity of the ether, one type predominates over the other.
- Substitutive nomenclature: the chain with the lowest number of carbons is named as the alkoxy substituent, followed by the name of the major hydrocarbon attached to the oxygen (recommendation C-211.2).
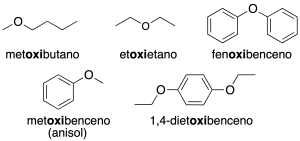
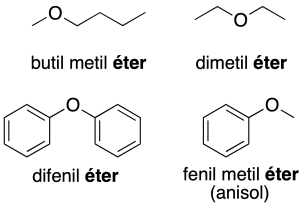
- Radical-functional nomenclature: the oxygen-bound radicals are named in alphabetical order, followed by the word ether (recommendation C-211.2).
Nomenclature of cyclic ethers
Cyclic ethers can be considered as heterocycles (rings with an atom other than carbon, in this case oxygen). For practical purposes they are classified into the following types:
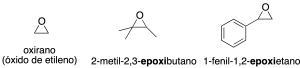
Epoxides or oxiranes (3 atoms)
They are oxygenated three-membered heterocycles. The simplest epoxide (C2H4O) is called oxirane, although it is also known as ethylene oxide. A common way of naming oxiranes is to use the prefix epoxy-, placing the grouping on a chain by the lowest combining locators (recommendations C-212.2 and R-5.5.4).
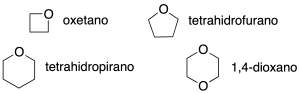
Cyclic ethers (4, 5 and 6 atoms)
They are named according to recommendations C-215 and B-1. In all cases the locator 1 is applied to the oxygen atom.
Crown ethers
They are cyclic polyethers that can be considered derivatives of ethylene glycol (1,2-ethanediol). The most widespread nomenclature system is specific only for this type of structures.
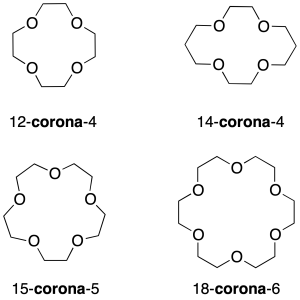
The first number in the name corresponds to the total number of atoms in the ring, followed by the word crown between hyphens and finally a second number indicating the total number of oxygens in the cycle.