What is Algar-Flynn-Oyamada reaction?
In 1934, the synthesis of flavones through oxidative cyclization of 2-oxychalcones with hydrogen peroxide under alkaline conditions was first reported by Algar and Flynn, and concurrently by Oyamada. This reaction is commonly known as the Algar-Flynn-Oyamada reaction, and has been referred to by several other names such as the Algar-Flynn oxidation, Algar-Flynn-Oyamada oxidation, AFO reaction, Algar-Flynn-Oyamada condensation, and AFO cyclization.
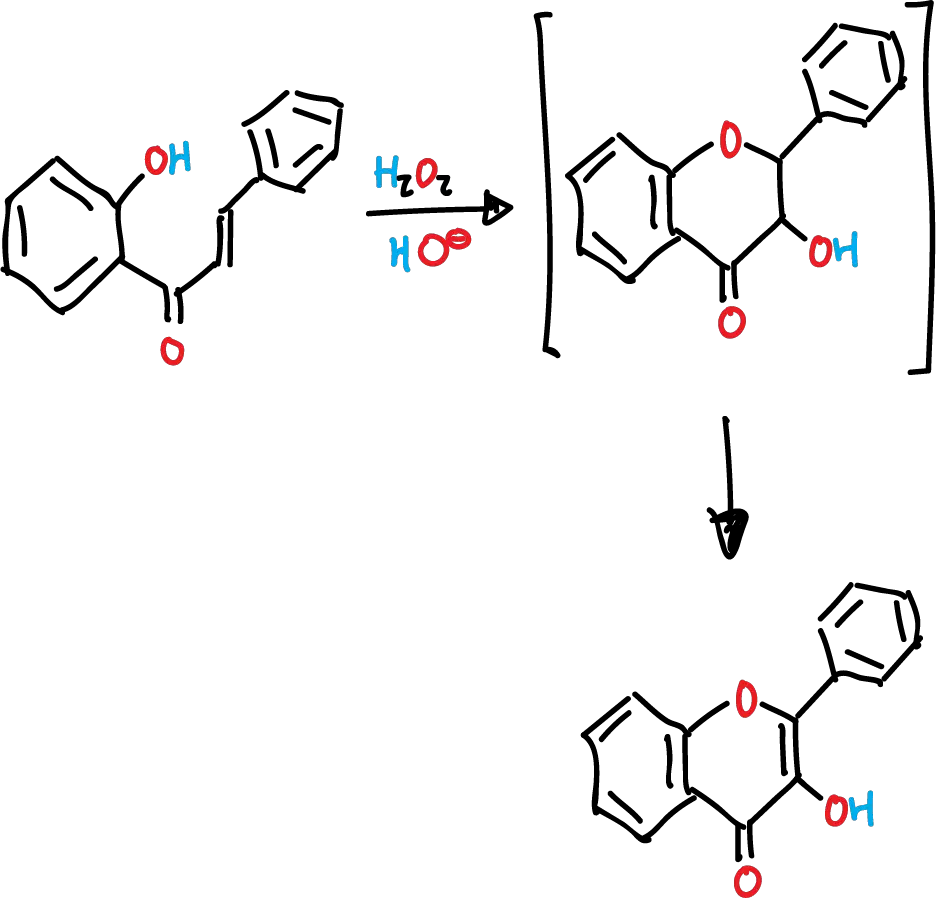
The Algar-Flynn-Oyamada reaction involves the alkaline oxidation of o-hydroxyphenyl styryl ketones (chalcones) using hydrogen peroxide, H2O2 as a reactant. This reaction results in the formation of flavonols via the intermediate dihydroflavonols.
Various 2-hydroxychalcones with methoxy groups in different positions in the two aromatic nuclei have been found to smoothly undergo the AFO oxidation.
References
- J. Algar, J.P. Flynn, “New synthesis of flavonols” Proc. Roy. Irish Acad. 42B, 1-8 (1934)
- T. Oyamada, A New General Method for the Synthesis of Flavonolderivatives, NIPPON KAGAKU KAISHI, 1934, Volume 55, Issue 12, Pages 1256-1260
DOI: 10.1246/nikkashi1921.55.12_1256
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.