What is Beckmann rearrangement?
The Beckmann rearrangement is a chemical reaction in which an oxime is converted into an amide through the use of an acid catalyst (acid-mediated isomerization). This reaction was first described by Ernst Otto Beckmann in 1886, and it has since become a widely used method for the synthesis of amides.
This reaction is known to cause ring enlargements when oximes of cyclic ketones are used.
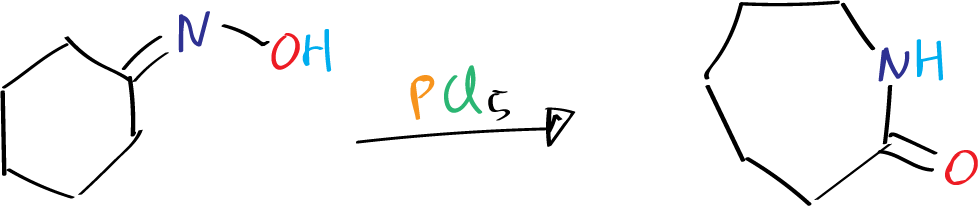
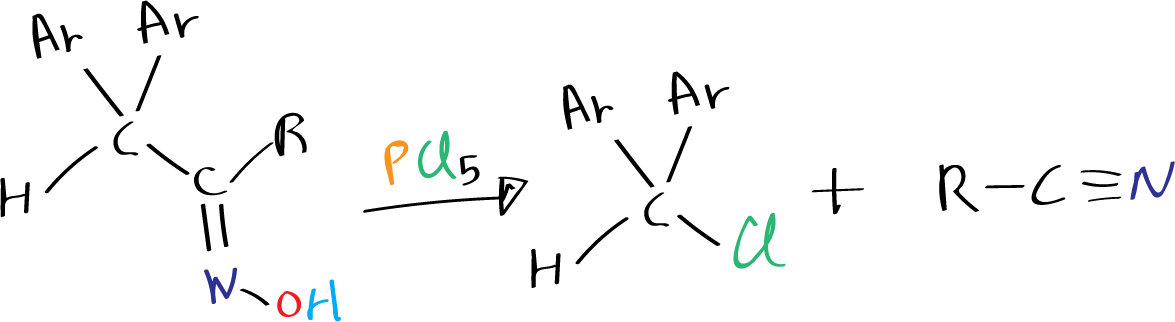
Some oximes, especially those with a quaternary carbon atom positioned opposite to the hydroxyl group, have a tendency to undergo Beckmann fragmentation to produce nitriles instead of amides.
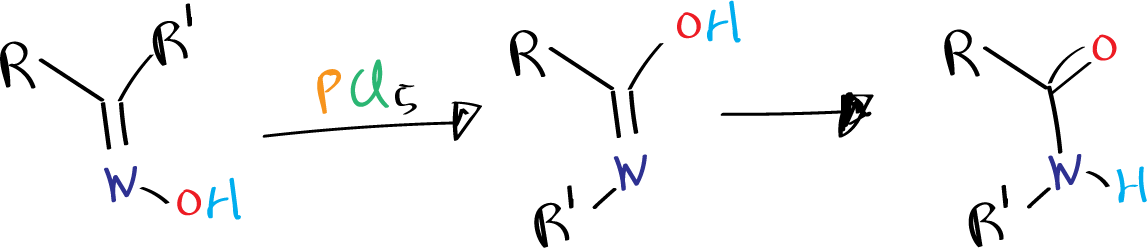
The general equation for the Beckmann rearrangement is as follows:
R-C(=N-OH)R’ + acid catalyst → R-C(=O)-NR’ + H2O
In this equation, R and R’ represent alkyl or aryl groups. The acid catalyst, which is typically sulfuric acid or hydrochloric acid, activates the oxime and facilitates the rearrangement of the molecule..
The Beckmann rearrangement is a useful method for the synthesis of amides because it allows for the introduction of an amide group into a molecule without the need for expensive and potentially hazardous reagents, such as dinitrogen monoxide (nitrous oxide). It is also a useful alternative to traditional methods for the synthesis of amides, such as the Curtius rearrangement or the Hofmann rearrangement..
There are several variations of the Beckmann rearrangement, including the use of different acid catalysts, such as trifluoroacetic acid or p-toluenesulfonic acid, and the use of different solvents, such as dichloromethane or acetone..
References
Beckmann, E. (1886), Zur Kenntniss der Isonitrosoverbindungen. [On the knowledge of isonitroso compounds.] Ber. Dtsch. Chem. Ges., 19: 988-993. https://doi.org/10.1002/cber.188601901222