What is Dimroth rearrangement?
The Dimroth rearrangement is a reaction first described by Dimroth in 1909 that involves the base-catalyzed translocation of exo– and endo-cyclic heteroatoms on a heterocyclic ring. This reaction is commonly referred to as the Dimroth rearrangement or the Dimroth-type rearrangement, and it is also known as the Dimroth reaction in some cases.
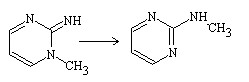
The Dimroth rearrangement occurs frequently on six-membered rings, although it can also occur on five-membered rings. Many of these rearrangements involve 1H-1,2,3-triazoles. The reaction involves a ring opening followed by a ring reclosure, and it typically results in the translocation of nitrogen as the heteroatom.
References
- Dimroth, O. (1909), Ueber intramolekulare Umlagerungen. Umlagerungen in der Reihe des 1,2,3-Triazols. [On intramolecular rearrangements. Rearrangements in the 1,2,3-triazole series.] Justus Liebigs Ann. Chem., 364: 183-226. https://doi.org/10.1002/jlac.19093640204
- Dimroth, O. and Michaelis, W. (1927), Intramolekulare Umlagerung der 5-Amino-1,2,3-triazole. [Intramolecular rearrangement of 5-amino-1,2,3-triazoles.] Justus Liebigs Ann. Chem., 459: 39-46. https://doi.org/10.1002/jlac.19274590104
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.