What is Fischer-Speier esterification?
In 1895, Fischer and Speier first described a reaction known as Fischer-Speier esterification. This involves refluxing a carboxylic acid with an excess of alcohol in the presence of an acidic catalyst, such as HCl, to produce an ester.
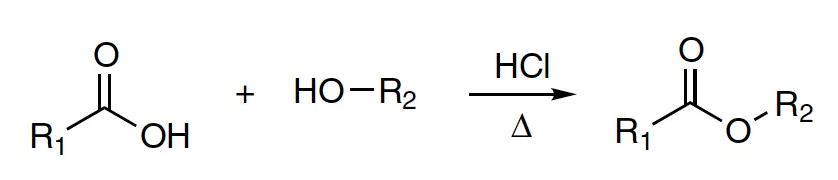
It is important to note that the reaction is reversible and that an equilibrium between the ester and carboxylic acid always exists. To drive the equilibrium towards the formation of ester, azeotropic refluxing is utilized to eliminate any newly formed water. To accelerate the esterification, an acidic catalyst such as dry HCl gas is added to the solution. This reaction is distinct from sulfuric acid-promoted esterification, as it does not produce olefins from the alcohol nor undergo electrophilic substitution with benzene. Methyl esters can be synthesized in almost quantitative yield by utilizing acetone dimethyl acetal as a water scavenger, which accelerates the reaction by generating two additional methanol molecules through the hydrolysis of acetone dimethyl acetal for every water molecule produced during esterification.
References
Fischer, E. and Speier, A. (1895), Darstellung der Ester. [Representation of the esters] Ber. Dtsch. Chem. Ges., 28: 3252-3258. https://doi.org/10.1002/cber.189502803176