What is Grignard reagents?
Organometallic compounds called Grignard reagents are widely utilized in organic synthesis due to their high reactivity. These reagents are synthesized through the reaction of a metal halide, such as methyl iodide, with magnesium turnings in an anhydrous ether solvent at room temperature. Their versatility is demonstrated by their common use in the synthesis of alcohols and other organic compounds.
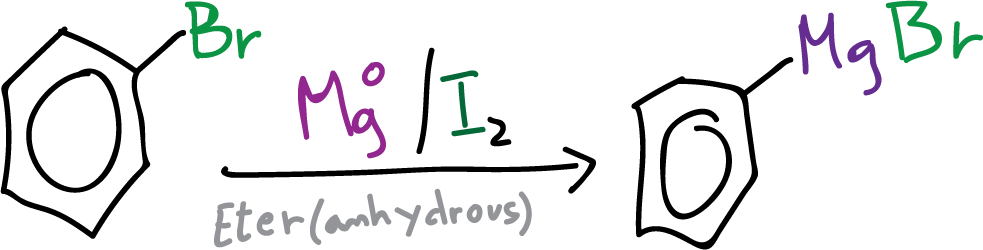
Notably, Grignard reagents can directly react with a ketone or an aldehyde without the need for isolation. When hydrolyzed with dilute acid, the corresponding tertiary or secondary alcohol can be produced in a much better yield than through the Barbier reaction.
References
Grignard, V. “Sur quelques nouvelles combinaisons organométalliques du magnesium et leur application à des Synthèses d’alcools et d’hydrocarbures” [On some new organometallic combinations of magnesium and their application to the synthesis of alcohols and hydrocarbons] Comptes Rendus Hebdomadaires des Séances de L’Academie des Sciences 1900, 130, 1322-1324.